In this issue of Blood, Braig et al have identified a novel mechanism of CD19-targeted immune escape.1
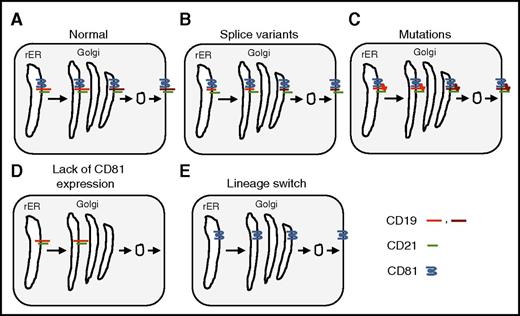
Mechanisms of CD19-targeted immune escape. The B-cell coreceptor complex consists of CD19, CD21, CD81, and CD225 (for simplicity CD225 is omitted here). It is assembled in the rough endoplasmic reticulum (rER) and transported to the Golgi apparatus where the carbohydrate moieties are processed before being transported to the cell surface. Carbohydrate processing is indicated only for CD19 (light red: unprocessed carbohydrates; dark red: processed carbohydrates). (A) Normal processing and transport of CD19. (B) Splice variants resulting in loss of the extracellular domain of CD19. (C) Mutations resulting in a conformational change of the extracellular domain of CD19. (D) Loss of CD81 expression resulting in intracellular accumulation of CD19 with unprocessed carbohydrates. (E) Lineage switch resulting in transcriptional silencing of CD19 expression.
Mechanisms of CD19-targeted immune escape. The B-cell coreceptor complex consists of CD19, CD21, CD81, and CD225 (for simplicity CD225 is omitted here). It is assembled in the rough endoplasmic reticulum (rER) and transported to the Golgi apparatus where the carbohydrate moieties are processed before being transported to the cell surface. Carbohydrate processing is indicated only for CD19 (light red: unprocessed carbohydrates; dark red: processed carbohydrates). (A) Normal processing and transport of CD19. (B) Splice variants resulting in loss of the extracellular domain of CD19. (C) Mutations resulting in a conformational change of the extracellular domain of CD19. (D) Loss of CD81 expression resulting in intracellular accumulation of CD19 with unprocessed carbohydrates. (E) Lineage switch resulting in transcriptional silencing of CD19 expression.
CD19-targeted therapies based on T cells that express CD19-specific chimeric antigen receptors (CARs) or CD19/CD3 bispecific T-cell engagers (blinatumomab) have potent antitumor activity in patients with CD19+ lymphoma and leukemia.2,3 However, CD19– immune escape has emerged as a major mode of therapeutic resistance, especially for patients with acute lymphoblastic leukemia (ALL). Splice variants, point mutations, and lineage switch have been described as part of the mechanism of CD19– immune escape (see figure)4-6 ; however, no complete deletion or gene silencing has been observed, most likely because of the critical role of CD19 signaling in B-cell malignancies.
In their article, Braig et al describe 4 patients with B-cell ALL (B-ALL) who developed CD19– relapse. All of them maintained a purely B-ALL phenotype, ruling out myeloid switch. For 1 patient, they performed a detailed analysis to elucidate the mechanism of immune evasion. In that patient, the CD19 gene had no mutations, and full-length CD19 messenger RNA was detected, excluding splice variants. On flow cytometric analysis, ALL blasts lacked expression of CD81 and CD21, two molecules that form the B-cell coreceptor complex with CD19 and CD225 on the cell surface of B cells. CD81 belongs to the transmembrane 4 superfamily of proteins,7 also known as the tetraspanin superfamily. It is widely expressed and plays a role in receptor signaling, cell adhesion, and myotube maintenance. In addition, it is the receptor for entry of human hepatitis C virus into hepatocytes. CD81 deficiency in humans primarily presents as humoral immunodeficiency, known as common variable immunodeficiency type 6.8 Studies in CD81 knockout mice have shown that CD81 associates with CD19 in the rER and is critical for transport of CD19 to the cell surface. The authors therefore examined the glycosylation status of CD19 and found that it is not fully glycosylated, indicating that this case of CD19– immune escape was the result of lack of CD81 expression, resulting in defective transport and/or maturation of CD19 in the ER and/or Golgi. More detailed studies are needed to determine the mechanism of CD81 deficiency because no mutations in the CD81 gene or transcriptional silencing were observed. At present, a defect in another gene cannot be excluded, since patients with CD81 deficiency have only a slight reduction of CD21 on their B cells8 in contrast to this patient’s CD19– ALL blasts, which lacked expression of CD81 and CD21.
Immune escape after adoptive T-cell therapy or peptide vaccinations has also been reported in clinical studies for Epstein-Barr virus–positive lymphoma, melanoma, or glioblastoma, highlighting the need to induce immune responses against a broad range of antigens to prevent immune escape. For example, transferring T-cell products that contain T cells expressing CARs with different specificity, expressing two CARs in a single T cell or a single CAR with 2 antigen-binding domains, or generating trispecific antibodies could potentially offset immune escape. However, approaches that rely solely on engineering multispecificity of infused T-cell products or antibodies might place us in the position of Achilles, who, according to Zeno’s paradox, was never able to catch the tortoise. Engineering T-cell products or antibodies to effectively induce antigen and/or epitope spreading seems to be an attractive strategy for overcoming this dilemma because this would induce the broadest, unbiased immune response against cancer. Indeed, several studies have already shown that adoptively transferred antigen-specific T cells can induce antigen/epitope spreading9 ; however, this requires further optimization.
In conclusion, the article by Braig et al adds to the increasing literature on how B-cell malignancies evade CD19-targeted therapies. Studies like this are critical for understanding the biology of immune escape and also have immense practical value. For example, developing a CD19/CD81 or CD19/CD21 dual targeting approach to circumvent CD19– immune escape would be ill-advised on the basis of the findings of Braig et al.
Conflict-of-interest disclosure: The Center for Cell and Gene Therapy at Baylor College of Medicine had a research collaboration with Celgene and Bluebird Bio, and currently has research collaborations with Cell Medica and Tessa Therapeutics. M.P.V. and S.G. have patents and/or patent applications in the fields of T-cell therapy and/or oncolytics.