Key Points
Ldb1 transcription factor self-association domain fused to γ-globin promoter-specific ZF protein increases HbF, reduces HbS in hSCD cells.
In vitro reactivation of HbF mediated by ZF-Ldb1 exceeds pharmacologic treatment in adult hSCD cells.
Abstract
Overcoming the silencing of the fetal γ-globin gene has been a long-standing goal in the treatment of sickle cell disease (SCD). The major transcriptional enhancer of the β-globin locus, called the locus control region (LCR), dynamically interacts with the developmental stage-appropriate β-type globin genes via chromatin looping, a process requiring the protein Ldb1. In adult erythroid cells, the LCR can be redirected from the adult β- to the fetal γ-globin promoter by tethering Ldb1 to the human γ-globin promoter with custom-designed zinc finger (ZF) proteins (ZF-Ldb1), leading to reactivation of γ-globin gene expression. To compare this approach to pharmacologic reactivation of fetal hemoglobin (HbF), hematopoietic cells from patients with SCD were treated with a lentivirus expressing the ZF-Ldb1 or with chemical HbF inducers. The HbF increase in cells treated with ZF-Ldb1 was more than double that observed with decitabine and pomalidomide; butyrate had an intermediate effect whereas tranylcypromine and hydroxyurea showed relatively low HbF reactivation. ZF-Ldb1 showed comparatively little toxicity, and reduced sickle hemoglobin (HbS) synthesis as well as sickling of SCD erythroid cells under hypoxic conditions. The efficacy and low cytotoxicity of lentiviral-mediated ZF-Ldb1 gene transfer compared with the drug regimens support its therapeutic potential for the treatment of SCD.
Introduction
Sickle cell disease (SCD) represents a major challenge and a growing health problem in Western countries, with ∼300 000 births every year.1 Altered deformability of sickle cells is caused by the polymerization of deoxygenated sickle hemoglobin (HbS), whose rate and extent are proportional to the extent and duration of hemoglobin (Hb) deoxygenation and the levels of fetal hemoglobin (HbF) in the erythrocyte.2 As a consequence, patients experience vaso-occlusive crises and red cell destruction in the peripheral circulation.3 Since the discovery that patients with hereditary persistence of HbF have a more benign clinical course,4 considerable research has been devoted toward approaches to augment HbF levels in adult red blood cells. Hydroxyurea (HU) is the only approved drug to induce HbF.5 Although its use is associated with marked clinical improvement, patients’ compliance and concerns about long-term effects hinder widespread utilization.6
Despite the improvement in the management of SCD, allogeneic hematopoietic stem cell transplantation remains the only curative option.7 New approaches from alternative donors showed promising results, however, they are limited to experimental protocols.8
Gene transfer using autologous bone marrow is a suitable option to cure SCD, and could resolve the issue of a matched donor and eliminate the risks of graft-versus-host disease.9 In recent clinical trials, the successful use of lentiviral vectors carrying the β- or γ-globin genes9,10 has opened the doors to other approaches, based on knockdown of γ-globin repressors and genome editing.11-13
Study design
We have developed another approach in which long-range enhancer-promoter contacts can be established by zinc finger (ZF)-mediated tethering of the “looping factor” Ldb1, leading to transcriptional activation.14 In murine and human adult erythroid cells from healthy individuals, this approach was successful in activating embryonic and fetal globin genes, respectively, thus partially reversing the developmental gene switch.15
The aim of this study was to compare the forced chromatin-looping strategy to known pharmacologic HbF inducers in adult erythroid cells from patients with SCD.
Results and discussion
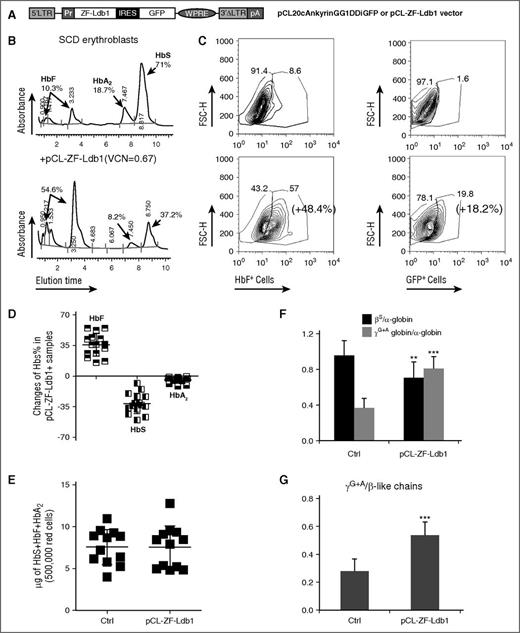
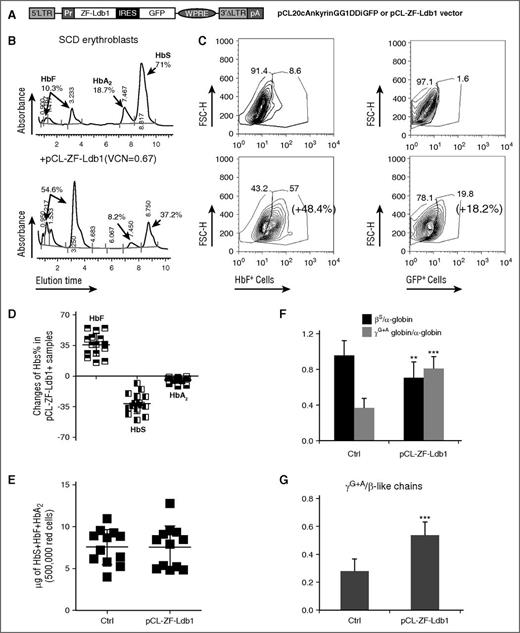
We aimed to improve HbF synthesis and the quality of erythroid cells derived from patients with SCD, using the lentiviral vector (pCL20cAnkyrinGG1DDiGFP), henceforth called pCL-ZF-Ldb1 (Figure 1A), which enabled Hb switch in healthy erythroblasts.15 As expected, sickle CD34+ cells produced predominantly HbS (α2βs2) once differentiated into erythroblasts in vitro (Figure 1B top). Other Hb, such as HbF (α2γ2) and HbA2 (α2δ2), were produced to a lower degree (Figure 1B top). Following infection with pCL-ZF-Ldb1 vector, the same SCD-derived erythroid cells increased the proportion of HbF and green fluorescent protein (GFP)-expressing erythroblasts (Figure 1C; supplemental Figure 1, available on the Blood Web site) and HbF from 10.3% (Figure 1B top) to 54.6% (Figure 1B bottom).
Increased HbF and reduced HbS synthesis after treatment with pCL-ZF-Ldb1. (A) Lentiviral construct carrying ZF-Ldb1 and the GFP genes expressed under the ankyrin promoter (Pr) via an internal ribosomal entry site (IRES). (B) Percentage of HbF, HbS, and HbA2 measured by high-performance liquid chromatography (HPLC) in representative erythroid cells untreated (top) or treated with ZF-Ldb1 (bottom). The transduced cells have, on average, 0.7 copies of viral molecules integrated per genome. (C) Changes in percentage of cells expressing HbF (left panels) and GFP (right panels), measured by flow cytometry. (D) Overall percentages of HbF, HbS, and HbA2 changes in SCD samples treated with pCL-ZF-Ldb1 (n = 10, 5 samples were duplicated; average VCN = 1.2). (E) Hb (F + S + A2) content in erythroid cells with or without pCL-ZF-Ldb1. (F) Ratio of β- or γ-globin chains over α-globin chains. The quantity of the globin chains (in μg) is measured via liquid chromatography under denaturing conditions (**P < .05). (G) Ratio of γ/β-like chains measured in 250 000 erythroid sickle cells untreated or treated with pCL-ZF-Ldb1 (n = 5, VCN = 1.6; ***P < .005 and **P < .05). FSC-H, forward scatter height; LTR, long terminal repeat; pA, polyadenylation signal; pCL, vector backbone; VCN, vector copy number; WPRE, woodchuck hepatitis virus posttranscriptional regulatory element.
Increased HbF and reduced HbS synthesis after treatment with pCL-ZF-Ldb1. (A) Lentiviral construct carrying ZF-Ldb1 and the GFP genes expressed under the ankyrin promoter (Pr) via an internal ribosomal entry site (IRES). (B) Percentage of HbF, HbS, and HbA2 measured by high-performance liquid chromatography (HPLC) in representative erythroid cells untreated (top) or treated with ZF-Ldb1 (bottom). The transduced cells have, on average, 0.7 copies of viral molecules integrated per genome. (C) Changes in percentage of cells expressing HbF (left panels) and GFP (right panels), measured by flow cytometry. (D) Overall percentages of HbF, HbS, and HbA2 changes in SCD samples treated with pCL-ZF-Ldb1 (n = 10, 5 samples were duplicated; average VCN = 1.2). (E) Hb (F + S + A2) content in erythroid cells with or without pCL-ZF-Ldb1. (F) Ratio of β- or γ-globin chains over α-globin chains. The quantity of the globin chains (in μg) is measured via liquid chromatography under denaturing conditions (**P < .05). (G) Ratio of γ/β-like chains measured in 250 000 erythroid sickle cells untreated or treated with pCL-ZF-Ldb1 (n = 5, VCN = 1.6; ***P < .005 and **P < .05). FSC-H, forward scatter height; LTR, long terminal repeat; pA, polyadenylation signal; pCL, vector backbone; VCN, vector copy number; WPRE, woodchuck hepatitis virus posttranscriptional regulatory element.
We analyzed the messenger RNA and Hb protein content of erythroblasts, untreated or after transduction with pCL-ZF-Ldb1, derived from 10 SCD subjects. On average, cells treated with pCL-ZF-Ldb1 produced nearly 40% more HbF compared with untreated cells (from 27.26% to 65.52%) and lowered production of HbS (−31.14%; from 62.83% to 31.69%) and HbA2 (−4.5%; from 9.5% to 5%) (Figure 1D). Messenger RNA content of γ-, β-, δ-globins and transgenic Ldb1 changed accordingly (supplemental Figure 2).
To assess the effect of ZF-Ldb1 treatment on the total amount of cellular Hb, we measured absolute Hb content in differentiated cells. Total Hb synthesis (HbF + HbS + HbA2) remained essentially unaltered (Figure 1E), regardless of significant shifts in the ratio of fetal to adult globin. Reversed-phase liquid chromatography, which allows for the quantification of single globin chains rather than tetrameric hemoglobin molecules, revealed that βS chains were diminished whereas both γA + γG chains were increased in specimens treated with ZF-Ldb1 (Figure 1F-G; supplemental Figure 3).
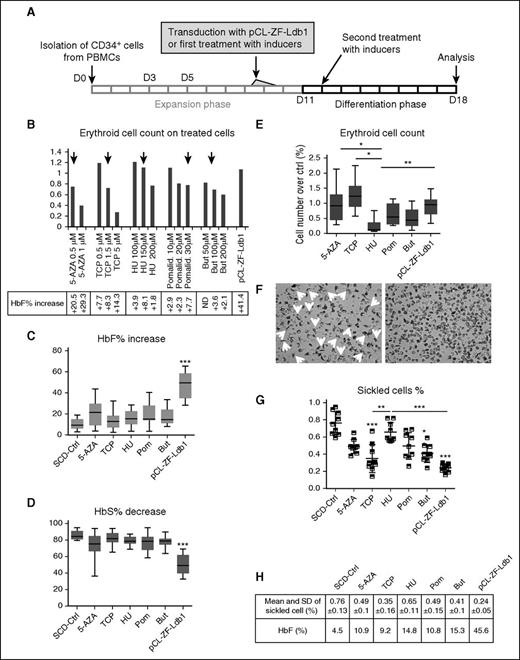
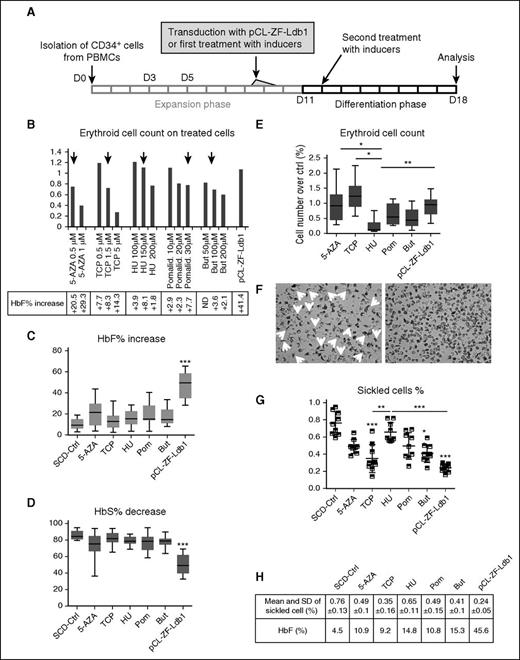
The experimental outline to compare pCL-ZF-Ldb1 to pharmacologic inducers of HbF is illustrated in Figure 2A. Briefly, SCD erythroid progenitor cells were infected with pCL-ZF-Ldb1 or treated with 5-aza-2′-deoxy-cytidine (0.5 μM), tranylcypromine (1.5 μM), HU (150 μM), pomalidomide (30μM), or butyrate (100 μM). Concentrations of inducers were determined through evaluation of efficacy (net increase of HbF) vs toxicity (cell death); the original scaling dosage (Figure 2B) was extrapolated from the most recent literature.16-18 Studies were performed at the end of phase 3 (day 18 from initial cell isolation), at the orthochromatophilic stage when Hb levels peaked. PCL-ZF-Ldb1–treated cells showed the most robust increase of HbF (Figure 2C) and decrease in HbS (Figure 2D). On average, the HbF in control (ctrl) SCD erythroblasts was 10.36% vs 21.05%, 14.16%, 15.98%, 18.91%, 17.34%, and 45.12% after treatment, respectively, with 5-aza-2′-deoxy-cytidine, tranylcypromine, HU, pomalidomide, butyrate, and ZF-Ldb1. In the same samples, on average, HbS decreased from 85.6%, in untreated SCD erythroblasts, to 74.8%, 81.7%, 79.1%, 76.9%, 78.1%, and 50.3% after treatments. ZF-Ldb1–expressing cells presented significantly higher variation in HbF and HbS than untreated and all other treated cells. Along with greater improvement of HbF levels, cells expressing ZF-Ldb1 did not show significant changes in viability compared with untreated samples, whereas cells treated with pomalidomide, butyrate, and HU showed reduced viability (Figure 2E). Moreover, the number of sickled cells in 3 independent samples treated with pCL-ZF-Ldb1 and exposed to hypoxic conditions was significantly reduced, also showing the least variability, when compared with controls or pharmacologically treated cells (Figure 2F-G). Taken together, pCL-ZF-Ldb1 was superior to all tested compounds in augmenting HbF and F-cell levels and importantly was associated with minimal toxicity.
Variation of HbF and HbS levels in CD34+-derived SCD erythroid cells treated with pCL-ZF-Ldb1 LV or HbF inducers in vitro. (A) Schematic representation of the experimental protocol of human SCD CD34+ cells treated with the inducer drugs or pCL-ZF-Ldb1. (B) Erythroid cell number (by benzidine staining; N = 2) using the indicated doses of drugs and compared with pCL-ZF-Ldb1 (with VCN ∼1 copy per cell). Black arrows indicate the dose of each drug chosen for the subsequent experiments. (C) Net increase of HbF percentage and (D) net decrease of HbS percentage in SCD erythroblasts (N = 7, 2 of which were replicated once or twice, respectively) treated with the HbF inducers or pCL-ZF-Ldb1 (***P < .005). (E) Erythroid cell count for each treatment normalized to count of untreated samples (*P = .05, **P < .05). (F) Morphological assessment of untreated (ctrl, left) vs pCL-ZF-Ldb1–treated cells (right) in hypoxic conditions. (G) Proportion of sickled cells/total cell count and (H) relative means and SD, measured on 3 replicas on 3 sets of samples treated with the various inducers or pCL-ZF-Ldb1 (**P < .05, ***P < .005) and exposed to hypoxic conditions. P values under and above bars refer to differences between treated and untreated samples and between treated samples, respectively. 5-AZA, 5-aza-2′-deoxy-cytidine; But, butyrate; ND, not determined; PBMC, peripheral blood mononuclear cell; Pom/Pomalid., pomalidomide; SD, standard deviation; TCP, tranyl-cypromine.
Variation of HbF and HbS levels in CD34+-derived SCD erythroid cells treated with pCL-ZF-Ldb1 LV or HbF inducers in vitro. (A) Schematic representation of the experimental protocol of human SCD CD34+ cells treated with the inducer drugs or pCL-ZF-Ldb1. (B) Erythroid cell number (by benzidine staining; N = 2) using the indicated doses of drugs and compared with pCL-ZF-Ldb1 (with VCN ∼1 copy per cell). Black arrows indicate the dose of each drug chosen for the subsequent experiments. (C) Net increase of HbF percentage and (D) net decrease of HbS percentage in SCD erythroblasts (N = 7, 2 of which were replicated once or twice, respectively) treated with the HbF inducers or pCL-ZF-Ldb1 (***P < .005). (E) Erythroid cell count for each treatment normalized to count of untreated samples (*P = .05, **P < .05). (F) Morphological assessment of untreated (ctrl, left) vs pCL-ZF-Ldb1–treated cells (right) in hypoxic conditions. (G) Proportion of sickled cells/total cell count and (H) relative means and SD, measured on 3 replicas on 3 sets of samples treated with the various inducers or pCL-ZF-Ldb1 (**P < .05, ***P < .005) and exposed to hypoxic conditions. P values under and above bars refer to differences between treated and untreated samples and between treated samples, respectively. 5-AZA, 5-aza-2′-deoxy-cytidine; But, butyrate; ND, not determined; PBMC, peripheral blood mononuclear cell; Pom/Pomalid., pomalidomide; SD, standard deviation; TCP, tranyl-cypromine.
Clinical observations indicate that the increase of HbF can ameliorate the severity of SCD. Although administration of HU in patients is associated with increased HbF levels, its therapeutic effects in children and adults are extremely variable, with increase of HbF ranging between 10% and 30%.6,19
Here, we investigated to which extent manipulation of the chromatin organization of the β-globin gene locus, via targeted tethering of the Ldb1 self-association domain to the β-like γG- and γA-globin promoters, can reverse the developmental gene switch in erythroblasts derived from SCD patients. We found that sickle erythroblasts treated with pCL-ZF-Ldb1 can yield up to a 40% increase of HbF synthesis and concurrently reduce HbS synthesis. Expression of ZF-Ldb1 in SCD led to 2 major beneficial outcomes, that is, increased HbF and reduced HbS content. Although the HbF content was 2.3-fold higher in treated erythroblasts, the overall Hb concentration, comprising HbF, HbS, and HbA2, remained unchanged, consistent with unimpeded erythroid maturation.
ZF-Ldb1 is a relatively small gene construct driven by a short promoter, which requires relatively low expression levels to activate the γ-globin genes. As small vectors significantly simplify production of lentiviral particles, this may also represent a considerable advantage over lentiviral vectors based on a classic additive gene-therapy approach, like those carrying curative β/γ-globin genes, which require additional large genomic elements to achieve therapeutic expression levels.20-23 Compared with gene editing and gene disruption, gene transfer for the cure of β-globinopathies has been shown to be safe in at least 10 patients.24,25 The therapeutic implication of our strategy for SCD patients has potential clinical application, as it not only sustains high levels of endogenous functional hemoglobin, but also reduces the quantity of sickled cells in hypoxic conditions.
In conclusion, a lentiviral vector carrying the self-association Ldb1 domain linked to a ZF protein, which selectively binds the γ-globin promoters, significantly increased HbF synthesis and exceeded the effects of previously described pharmacologic inducers in human cultured cells. Further lines of investigation will provide additional insight on the impact of this approach in vivo and on its safety and applicability for clinical purposes.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work has been supported by National Institutes of Health (NIH) National Center for Advancing Translational Sciences grant KL2TR000458 (L.B.); NIH National Heart, Lung, and Blood Institute (NHLBI) grants 5R01HL102449 (S.R.) and 5R01HL119479 (G.A.B.); European Community grant FP7-HEALTH-2012-INNOVATION (S.R.); and the Associazione Veneta per la Lotta alla Thalassemia (AVLT, IT; L.B. and S.R.). Additional funding was provided by NIH NHLBI grant 5K01HL103186, and NIH National Institute of Diabetes and Digestive and Kidney Diseases grant R01DK084188 (O.Y.A.). The authors gratefully acknowledge the generous support by the Jean and DiGaetano families and the Children’s Hospital of Philadelphia Foundation.
Authorship
Contribution: L.B., S.R., I.M. and G.A.B. designed research, analyzed and interpreted data, and wrote the paper; L.B., I.M., C.G., S.L., and O.Y.A. performed experiments and collected data; and D.M., W.D., and J.W.R. contributed vital new reagents and contributed to the editing of the paper.
Conflict-of-interest disclosure: S.R. is a consultant for Novartis, Ionis and Keryx Pharmaceuticals, Nektar and Rana Therapeutics, and Merganser Biotech. In addition, S.R. is a co-inventor for the patents US8058061 B2 C12N 20111115 and US7541179 B2 C12N 20090602. The consulting work and intellectual property of S.R. did not affect in any way the design, conduct, or reporting of this research. The remaining authors declare no competing financial interests.
Correspondence: Laura Breda, Department of Pediatrics, Division of Hematology, Children’s Hospital of Philadelphia, 3615 Civic Center Blvd, Room 302B, Philadelphia, PA, 19104; e-mail: bredal@email.chop.edu.
References
Author notes
G.A.B. and S.R. share senior co-authorship.