Key Points
CML LSC demonstrate increased IL-1 receptor expression and enhanced signaling response.
Inhibition of IL-1 signaling using the antagonist IL-1RA enhances targeting of CML LSC in combination with TKI.
Abstract
Treatment of chronic myelogenous leukemia (CML) with BCR-ABL tyrosine kinase inhibitors (TKI) fails to eliminate leukemia stem cells (LSC). Patients remain at risk for relapse, and additional approaches to deplete CML LSC are needed to enhance the possibility of discontinuing TKI treatment. We have previously reported that expression of the pivotal proinflammatory cytokine interleukin-1 (IL-1) is increased in CML bone marrow. We show here that CML LSC demonstrated increased expression of the IL-1 receptors, IL-1 receptor accessory protein and IL-1 receptor type 1 (IL-1R1), and enhanced sensitivity to IL-1-induced NF-κB signaling compared with normal stem cells. Treatment with recombinant IL-1 receptor antagonist (IL-1RA) inhibited IL-1 signaling in CML LSC and inhibited growth of CML LSC. Importantly, the combination of IL-1RA with TKI resulted in significantly greater inhibition of CML LSC compared with TKI alone. Our studies also suggest that IL-1 signaling contributes to overexpression of inflammatory mediators in CML LSC, suggesting that blocking IL-1 signaling could modulate the inflammatory milieu. We conclude that IL-1 signaling contributes to maintenance of CML LSC following TKI treatment and that IL-1 blockade with IL-1RA enhances elimination of TKI-treated CML LSC. These results provide a strong rationale for further exploration of anti-IL-1 strategies to enhance LSC elimination in CML.
Introduction
Chronic myelogenous leukemia (CML) results from hematopoietic stem cell (HSC) transformation by the BCR-ABL kinase.1 CML is characterized by a myeloproliferative disorder that progresses from initial chronic phase (CP) to accelerated phase (AP) and terminal blast crisis (BC). BCR-ABL tyrosine kinase inhibitors (TKI) are remarkably effective in inducing remissions, preventing transformation to BC, and prolonging survival of CML patients.2 However, TKI treatment fails to eliminate CML leukemia stem cells (LSC), even in patients achieving deep molecular responses.3 Although a subset of CML CP patients are able to maintain remission after stopping TKIs, most patients require continued treatment to prevent relapse.4,5 The risks of prolonged treatment include toxicity and considerable cost, which may affect adherence to treatment.6 Improved understanding of LSC resistance to TKI could help guide development of strategies to increase the proportion of CML patients achieving treatment-free remissions (TFR).
We have studied LSC in CML using primary blood and bone marrow (BM) samples from CP CML patients as well as an inducible transgenic mouse model of CML in which BCR-ABL is expressed under control of a Tet-regulated 3′ enhancer of the murine stem cell leukemia (SCL) gene.7 Because in vivo characterization of human CML CP LSC in immunodeficient mice is limited by low levels of engraftment and lack of leukemia development, the transgenic mouse model has proven very useful to study CML LSC regulation and therapeutic strategies.8 We have found that leukemia-induced alterations in the BM microenvironment provide a competitive advantage to CML compared with normal long-term HSC (LTHSC). Enzyme-linked immunosorbant assays (ELISA) demonstrate increased expression of the pivotal proinflammatory cytokines interleukin-1α (IL-1α) and IL-1β in the BM of BCR-ABL mice. IL-1α and IL-1β significantly increased growth of CML compared with normal LTHSC. BM cells from CML patients also demonstrated increased expression of IL-1α, but not IL-1β, compared with normal BM cells. IL-1α significantly increased expansion of CML compared with normal CD34+CD38− cells. Interestingly, IL-1α expression remained elevated in BM from CML patients in long-term remission on imatinib treatment.9
IL-1 signals through the IL-1 receptor type 1 (IL-1R1) and IL-1 receptor accessory protein (IL-1RAP, IL-1R3) and activates the NF-κB, JNK, and p38 MAPK pathways. IL-1 signaling is enhanced in certain tumors and associated with aggressive phenotype.10 Järås et al observed that IL-1RAP is selectively overexpressed in Philadelphia-positive +) CML stem cells, and that an anti-IL-1RAP antibody could target CML CD34+CD38− cells via antibody-dependent cell-mediated cytotoxicity.11 However, the role of IL-1 signaling in TKI resistance of CML LSC is not known, and it is not known whether inhibiting IL-1 signaling could enhance elimination of CML LSC. IL-1 receptor antagonist (IL-1RA) is a member of the IL-1 cytokine family that binds nonproductively to IL-1R1, preventing IL-1α and IL-1β signaling. Recombinant human IL-1RA (Anakinra, Kinaret) is clinically approved for the treatment of rheumatoid arthritis.12 Here, we evaluated the role of IL-1 signaling in CML LSC using IL-1RA, and studied its ability to modulate their sensitivity to TKI treatment.
Methods
Mice
SCL-tTA/BCR-ABL mice in the CD45.1 FVB/N background7 were maintained on tetracycline. Recipient mice were in the CD45.2 FVB/N background (kind gift from Emmanuelle Passegue, University of California, San Francisco). CD45.1 SCL-tTA/BCR-ABL mice crossbred with CD45.2 mice to generate CD45.1.2 SCL-tTA/BCR-ABL mice were used as recipients for one experiment. Mouse care and experimental procedures followed federal guidelines, and protocols were approved by the Institutional Animal Care and Use Committee at the City of Hope.
Samples
Cord blood (CB) samples were provided by StemCyte (Arcadia, CA). Aliquots of normal peripheral blood (PB) and BM mononuclear cells (MNC) were obtained from transplant donors. CML BM samples were obtained from patients in CP who had not received TKI treatment from City of Hope and the University of Glasgow. Sample acquisition was approved by the institutional review boards at City of Hope and the North Glasgow University Hospital Division of National Health Service Greater Glasgow and Clyde in accordance with the Declaration of Helsinki. All donors signed informed consent forms. MNC were isolated using Ficol separation. CD34+ cells were isolated using magnetic bead selection protocol (Miltenyi).
Conditioned medium
To make conditioned medium (CM), 20 million fresh human or murine CML or normal MNCs were cultured in 10 mL StemSpan serum-free expansion medium for 24 hours, and the supernatant was collected as CM.
In vivo treatment of transgenic BCR-ABL mice
BM cells from BCR-ABL mice (CD45.1) were transplanted by tail vein into CD45.2 recipient mice (2 × 106 cells/mouse) irradiated at 900 cGy. Blood samples were obtained after 4 weeks to confirm development of neutrophilic leukocytosis. Mice were treated with nilotinib (NIL; 50 mg/kg/d, oral gavage; Novartis, Basel, Switzerland), IL-1RA (150 mg/kg/d, subcutaneously; Anakinra; Sobi, Stockholm, Sweden), NIL+IL-1RA, or vehicle for 3 weeks, euthanized, and PB, BM, and spleen cells were obtained. The IL-1RA dose was based on prior reports indicating that this dose inhibited IL-1 effects in mice.13 Donor-derived CD45.1+ myeloid, progenitor, and stem cell populations were measured by flow cytometry. Long-term hematopoietic stem cells (LTHSC, LSK Flt3−CD150+CD48−), multipotent progenitors (MPP, LSK Flt3−CD150−CD48−, LSK Flt3−CD150+CD48+, LSK Flt3−CD150−CD48+), common myeloid progenitors (CMP, Lin−Sca-1−c-Kit+CD34+Fc RII/IIIlo), and granulocyte-macrophage progenitors (GMP, Lin−Sca-1−c-Kit+CD34+Fc RII/IIIhi) were identified using flow cytometry. Pooled BM cells from treated mice were transplanted into CD45.2 mice (2 × 106cells/mouse). Engraftment in PB was monitored every 4 weeks. The fraction of mice developing leukemia, manifested by neutrophilic leukocytosis, within 16 weeks was determined, and survival was monitored. Another cohort of mice was treated with NIL or NIL+IL-1RA for 4 weeks and monitored for survival after treatment discontinuation.
In vitro proliferation, apoptosis, colony forming assays
Human CD34+ cells were labeled with 1.25 μM carboxyfluorescein diacetate succinimidyl ester (CFSE; Molecular Probes, Eugene, OR) and antibodies, and flow cytometry was sorted to collect CD34+CD38− and CD34+CD38+ cells with uniform CFSE labeling.14 Cells were cultured with human normal or CML BM CM with IL-1RA (5 µg/mL), NIL (5 µM), or the combination for 72 hours. Cell numbers were measured by Lumino Glo (Promega). To measure apoptosis and proliferation, cells were labeled with Annexin V (Becton Dickenson [BD], San Diego, CA) and analyzed by flow cytometry for Annexin V and CFSE fluorescence.14 ModFit software (Verity, Topsham, ME) was used to assess the different cell generations and proliferation index. For colony-forming cell (CFC) assays, cells were plated in methylcellulose progenitor culture burst-forming-units-erythroid and colony-forming-units-granulocyte and macrophage counted after 14 days. Colony replating assays were performed by collecting and pooling colonies from primary CFC assays and plating 10 000 cells in secondary CFC assays.
Engraftment of human cells in immunodeficient mice
CML CD34+ cells (1 × 106 cells/mouse) or CB CD34+ cells (1 × 105 cells/mouse) were cultured in CML CM for 72 hours with NIL (5 μM), IL-1RA (5 µg/mL), or the combination, and transplanted via tail vein into sublethally irradiated (300 cGy) 8-week-old NOD.Cg-PrkdcscidIL2rgtm1Wjl/SzJ mice (NSG mice; The Jackson Laboratory, Bar Harbor, ME). Mice were euthanized after 4 or 16 weeks, and BM and blood cells were obtained at necropsy. Human CD45-expressing cells were enumerated by flow cytometry. Quantitative polymerase chain reaction (qPCR) analysis was performed using primer and probe sequences for BCR-ABL (B3A2 or B2A2). Human BCR levels were measured as internal controls.
IL-1 receptor and signaling assays
IL-1R1 (R&D Systems, cat: FAB269A) and IL-1 receptor accessory protein (IL-1RAP; R&D Systems, cat: FAB676P) expression was measured by flow cytometry. To assess IL-1 signaling, CD34+ cells from CB, normal BM, and CML BM were cultured in serum-free expansion medium with IL-1α (10 ng/mL; Peprotech, cat: 200-01A), or in CML or normal BM CM, for 16 hours, and p-NF-κB (p65) (BD, cat: 558422), p-p38 MAPK (BD, cat: 612594), and p-JNK (BD, cat: 562480) expression was measured by flow cytometry. To measure the effects of IL-1RA and NIL, CML Lin-CD34+CD38−CD90+ cells were cultured in CML BM CM with IL-1RA (5 µg/mL), NIL (5 µM), or the combination for 24 hours. Cells were analyzed by flow cytometry for p-CrkL (Cell Signaling, cat: 3181), p-NF-κB, p-p38 MAPK, and p-JNK expression. Results were expressed as a ratio of median channel fluorescence to isotype controls.
qRT-PCR analysis
Human CML BM CD34+CD38−CD90+ cells were cultured in CML CM with IL-1RA (5 µg/mL), NIL (5 µM), or the combination for 24 hours. RNA was extracted and first-strand cDNA synthesized from 200 cells, preamplified for 18 cycles, and multiplex qRT-PCR performed using TaqMan Assays (Thermo Fisher Scientific, Waltham, MA; Table 1) and the Fluidigm Biomark system. Results were expressed as ratio to β2m.
Analysis of microarray data
Data were obtained from GEO (GSE12211) and normalized using affy::rma in R.15 Differential expression was calculated using LIMMA, using a paired-sample design. Gene annotations were obtained from hgu133a2.db library in R. Gene set enrichment analysis (GSEA) was performed using GSEA software (Broad Institute).
Immunofluorescence analysis
CML CD34+CD38−CD90+ cells cultured in CML CM with NIL (5 µM), IL-1RA (5 µg/mL), or the combination for 24 hours were spun onto glass slides. Cells were fixed with 4% paraformaldehyde, permeabilized with 0.1% Triton X-100, and incubated with RelA/NF κB p65 antibody (MAB5078; R&D Systems), and FITC-conjugated anti-mouse secondary antibodies. Slides were examined using a Zeiss upright LSM 510 2-photon confocal microscope.
ELISA
ELISA was performed using R&D Systems human IL-1α Quantikine ELISA kit (DLA50), according to the manufacturer’s protocol.
Statistics
Data were reported as the mean ± standard error of the mean (SEM). Student t test and 1- or 2-way analysis of variance (ANOVA) with multiple testing were performed to determine statistical significance, as appropriate. P < .05 was considered statistically significant.
Results
IL-1RA inhibits growth of murine CML stem cells
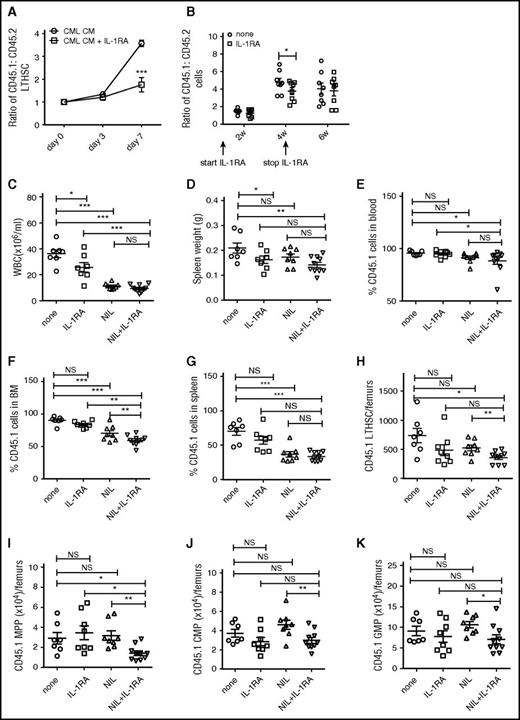
We have previously shown that CML LTHSC have a significant growth advantage over normal LTHSC when cultured in CML compared with normal BM CM.9 To evaluate the role of IL-1 within CML BM CM in regulating CML LTHSC growth, LTHSC from SCL-tTA/BCR-ABL mice (CD45.1) were mixed in a 1:1 ratio with LTHSC from congenic FVB/N mice (CD45.2) and cultured for 7 days with CML BM CM with and without IL-1RA to block IL-1 receptor signaling. The ratio of CML to normal LTHSC was significantly decreased by IL-1RA (Figure 1A), indicating that IL-1RA reduced the growth advantage of CML LTHSC.
IL-1RA inhibits growth of murine CML stem cells and enhances elimination of CML stem cells in combination with NIL. CML (CD45.1) and normal (CD45.2) LTHSC were mixed with in a 1:1 ratio and cultured in murine CML BM CM with or without IL-1RA (2.5 µg/mL) for 7 days. The ratio of CD45.1+ to CD45.2+ cells is shown (A). BM cells from CML (CD45.1) and control (CD45.2) mice were mixed in a 1:1 ratio and transplanted into irradiated CML recipient mice (CD45.1.2). The recipient mice were treated with or without IL-1RA (150 mg/kg/d, subcutaneously) for 4 weeks. The ratio of CML to control cells is shown in (B). BM cells from BCR-ABL mice (CD45.1) were transplanted by tail vein injection (2 × 106 cells/mouse) into recipient mice (CD45.2). Mice were treated with IL-1RA (150 mg/kg/d, subcutaneously), NIL (50 mg/kg/d, oral gavage), IL-1RA+NIL, or vehicle for 3 weeks (n = 10 each). PB WBC count (C); spleen weight (D); percentage CD45.1+ cells in PB (E), BM (F) and spleen (G); and number of LTHSC (H), MPP (I), CMP (J) and GMP (K) in the BM are shown. Results represent mean ± SEM. Significance values: *P < .05; **P < .01; ***P < .001, 2-way ANOVA. NS, not significant.
IL-1RA inhibits growth of murine CML stem cells and enhances elimination of CML stem cells in combination with NIL. CML (CD45.1) and normal (CD45.2) LTHSC were mixed with in a 1:1 ratio and cultured in murine CML BM CM with or without IL-1RA (2.5 µg/mL) for 7 days. The ratio of CD45.1+ to CD45.2+ cells is shown (A). BM cells from CML (CD45.1) and control (CD45.2) mice were mixed in a 1:1 ratio and transplanted into irradiated CML recipient mice (CD45.1.2). The recipient mice were treated with or without IL-1RA (150 mg/kg/d, subcutaneously) for 4 weeks. The ratio of CML to control cells is shown in (B). BM cells from BCR-ABL mice (CD45.1) were transplanted by tail vein injection (2 × 106 cells/mouse) into recipient mice (CD45.2). Mice were treated with IL-1RA (150 mg/kg/d, subcutaneously), NIL (50 mg/kg/d, oral gavage), IL-1RA+NIL, or vehicle for 3 weeks (n = 10 each). PB WBC count (C); spleen weight (D); percentage CD45.1+ cells in PB (E), BM (F) and spleen (G); and number of LTHSC (H), MPP (I), CMP (J) and GMP (K) in the BM are shown. Results represent mean ± SEM. Significance values: *P < .05; **P < .01; ***P < .001, 2-way ANOVA. NS, not significant.
To analyze effects of IL-1RA on in vivo growth of CML LSC, BM cells from SCL-tTA/BCR-ABL and congenic FVB/N mice were mixed in a 1:1 ratio and transplanted into irradiated BCR-ABL recipient mice (CD45.1.2). BCR-ABL expression was induced in recipient mice for 4 weeks prior to transplant, to generate leukemia-related changes in the BM microenvironment. Mice were treated with IL-1RA or vehicle. The ratio of CML to normal cells was significantly reduced in PB of IL-1RA–treated compared with control mice after 4 weeks (Figure 1B). Subsequent withdrawal of IL-1RA resulted in return of CML to normal cell ratios to levels similar to controls.
IL-1RA enhances elimination of murine CML stem cells treated with NIL
To test the effect of IL-1RA by itself and in combination with NIL on CML LTHSC in vivo, BM cells from SCL-tTA/BCR-ABL mice (CD45.1) were transplanted into congenic FVB/N mice (CD45.2) to generate a cohort of mice with CML-like disease. This approach allows the generation of a large number of mice with similar time of onset of leukemia, facilitating studies of therapeutic interventions against CML stem cells.8 Following CML development 4 weeks after transplantation, mice were treated with IL-1RA, NIL, or the combination for 3 weeks. White blood cell (WBC) counts were reduced after treatment with IL-1RA, NIL, and the combination (Figure 1C). IL-1RA and the IL-1RA plus NIL combination reduced spleen cellularity (Figure 1D). The combination of NIL and IL-1RA significantly reduced the percentage of engraftment of CD45.1+ CML cells in PB (Figure 1E), BM (Figure 1F), and spleen (Figure 1G) and further reduced CML engraftment in BM, but not in blood and spleen, compared with NIL alone. IL-1RA or NIL alone did not reduce CML BM LTHSC (Figure 1H) and MPP (Figure 1I), whereas the combination of IL-1RA and NIL significantly reduced CML LTHSC and MPP compared with controls and NIL alone. Finally, IL-1RA, NIL, and the combination did not reduce CML CMP (Figure 1J) and GMP (Figure 1K) in the BM. IL-1RA did not significantly affect the number of CML LTHSC (supplemental Figure 1A, available on the Blood Web site), CMP (supplemental Figure 1C), and GMP (supplemental Figure 1D) in the spleen, although MPPs (supplemental Figure 1B) were reduced. NIL and the combination of NIL plus IL-1RA did not affect LTHSC, but significantly reduced MPP, CMP, and GMP in the spleen of CML mice (supplemental Figure 1A-D). Thus, combination treatment results in enhanced inhibition of BM cells, particularly stem and progenitor populations, but does not significantly inhibit mature cells in blood and spleen or splenic LTHSC.
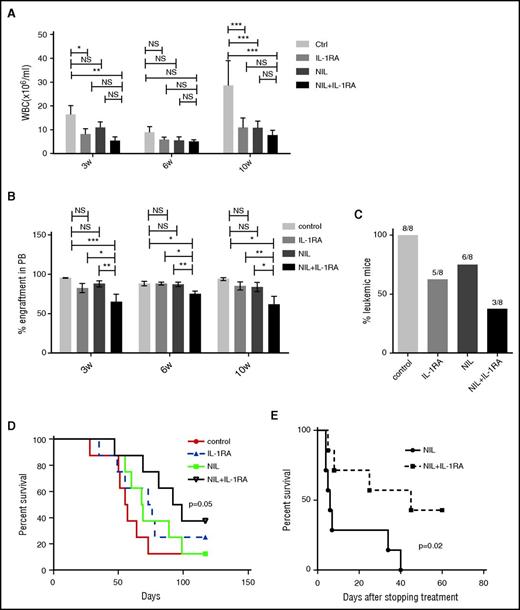
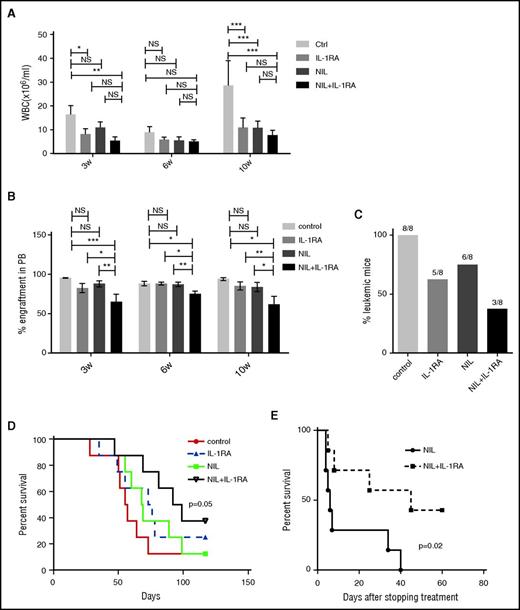
Secondary transplantation of BM cells from CML mice (CD45.1) treated with NIL plus IL-1RA into wild-type recipient mice (CD45.2) was associated with significantly reduced WBC counts (Figure 2A), reduced percentage of CML cell engraftment (Figure 2B), reduced leukemia development (Figure 2C), and significantly prolonged survival, compared with untreated controls (Figure 2D). Mice treated with NIL or the combination of NIL and IL-1RA for 4 weeks were also followed for survival. Mice treated with both IL-1RA and NIL showed markedly improved survival after stopping treatment (Figure 2E). These results indicate that addition of IL-1RA to NIL enhances inhibition of BM LSC capable of engrafting secondary recipients and causing disease relapse.
IL-1RA enhances elimination of CML stem cells in combination with NIL. BM cells from CML mice (CD45.1) treated with IL-1RA alone, NIL alone, or IL-1RA in combination with NIL were transplanted into irradiated secondary recipient mice (CD45.2) (n = 8 each). WBC counts (A), percent CD45.1 engraftment in PB (B), frequency of the mice developing leukemia (C), and survival (D) of the secondary recipient mice are shown. Another cohort of mice (n = 7 each) was treated with NIL alone or combination of NIL and IL-1RA for 4 weeks following which treatment was stopped, and the survival of these mice are shown (D). Results represent mean ± SEM. Significance values: *P < .05; **P < .01, ***P < .001, 2-way ANOVA and log-rank test.
IL-1RA enhances elimination of CML stem cells in combination with NIL. BM cells from CML mice (CD45.1) treated with IL-1RA alone, NIL alone, or IL-1RA in combination with NIL were transplanted into irradiated secondary recipient mice (CD45.2) (n = 8 each). WBC counts (A), percent CD45.1 engraftment in PB (B), frequency of the mice developing leukemia (C), and survival (D) of the secondary recipient mice are shown. Another cohort of mice (n = 7 each) was treated with NIL alone or combination of NIL and IL-1RA for 4 weeks following which treatment was stopped, and the survival of these mice are shown (D). Results represent mean ± SEM. Significance values: *P < .05; **P < .01, ***P < .001, 2-way ANOVA and log-rank test.
IL-1RA inhibits growth of human CML stem cells treated with NIL
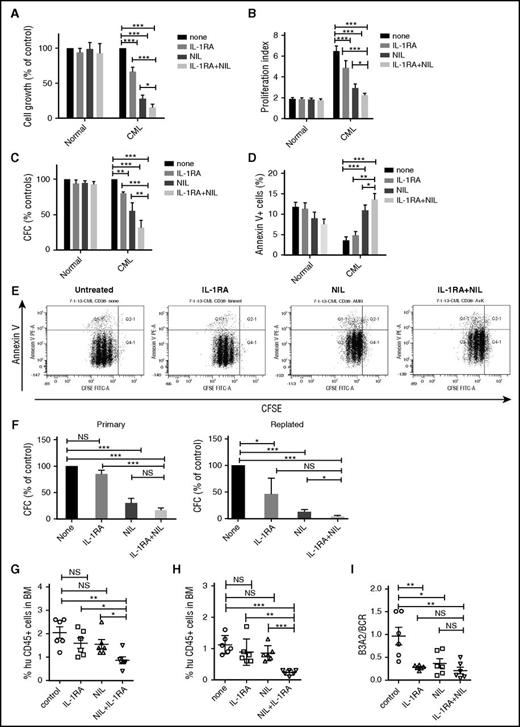
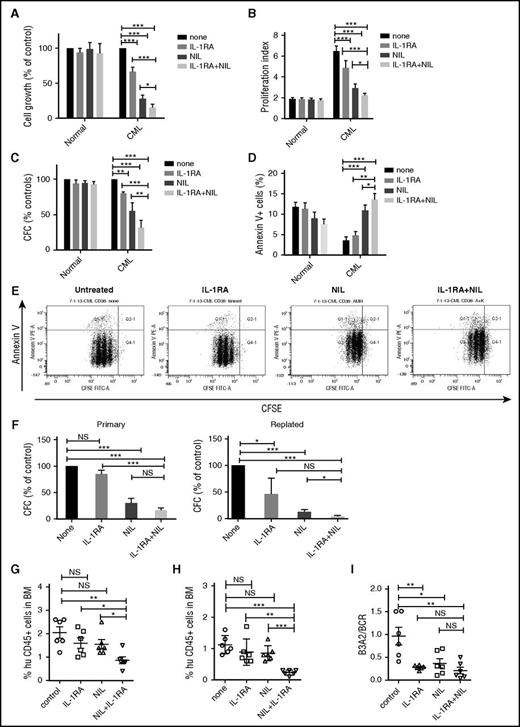
To study the effect of IL-1RA on human CML stem and progenitor cells, CML and normal CD34+CD38− stem/progenitor cells and CD34+CD38+ committed progenitors were cultured in CML BM CM and exposed to NIL (5 µM), IL-1RA (5 µg/mL), or NIL+IL-1RA for 72 hours. A dose-response analysis showed that the IL-1RA concentration used achieved maximal in vitro inhibitory effects (supplemental Figure 2A). IL-1RA significantly inhibited CML CD34+CD38− cell growth (Figure 3A), cell division (Figure 3B,E), and CFC frequency (Figure 3C) and increased apoptosis (Figure 3D,E), compared with NIL or IL-1RA alone. The combination of NIL and IL-1RA also significantly reduced CML CD34+CD38+ cell growth (supplemental Figure 2B), cell division (supplemental Figure 2C), and CFC frequency (supplemental Figure 2D), but did not change apoptosis, compared with NIL alone (supplemental Figure 2E). The combination of IL-1RA and NIL significantly inhibited replating capacity of CML CFC compared with controls and NIL alone (Figure 3F). In contrast, no significant inhibition of cell growth, cell division, and CFC frequency, or increase in apoptosis, was seen in normal CD34+CD38− (Figure 3A-D) or CD34+CD38+ cells (supplemental Figure 2B-E) treated with NIL, IL-1RA, or the combination.
IL-1RA inhibits growth of human CML stem cells in combination with NIL. Primary human CML and normal CD34+CD38− primitive stem/progenitor cells were cultured in human CML BM CM and exposed to NIL (5 μM), IL-1RA (5 μg/mL), NIL+IL-1RA, or vehicle for 72 hours. Cell growth (A), cell proliferation (B), CFC (C), and apoptosis (D) are shown. A representative flow cytometry plot showing CFSE and Annexin V labeling is shown (E). CD34+ cells treated with NIL, IL-1RA, and NIL+IL-1RA were plated in CFC assays (left), and after 14 days colonies were harvested and cells resuspended and replated in secondary CFC assays (right) (F). CML CD34+ cells treated with IL-1RA, NIL, or IL-1RA in combination with NIL for 72 hours were transplanted into irradiated (300 cGy) NSG mice (n = 6 each). Engraftment of human CD45+ cells in the BM of NSG mice at 4 weeks (G) and 16 weeks (H) is shown. BCR/ABL expression in the enriched human CD45+ cells in the BM at 16 weeks was measured (I). Results represent mean ± SEM. Significance values: *P < .05; **P < .01; ***P < .001, 2-way ANOVA, 1-way ANOVA. FITC-A, fluorescein isothiocyanate-A; PE-A, phycoerythrin.
IL-1RA inhibits growth of human CML stem cells in combination with NIL. Primary human CML and normal CD34+CD38− primitive stem/progenitor cells were cultured in human CML BM CM and exposed to NIL (5 μM), IL-1RA (5 μg/mL), NIL+IL-1RA, or vehicle for 72 hours. Cell growth (A), cell proliferation (B), CFC (C), and apoptosis (D) are shown. A representative flow cytometry plot showing CFSE and Annexin V labeling is shown (E). CD34+ cells treated with NIL, IL-1RA, and NIL+IL-1RA were plated in CFC assays (left), and after 14 days colonies were harvested and cells resuspended and replated in secondary CFC assays (right) (F). CML CD34+ cells treated with IL-1RA, NIL, or IL-1RA in combination with NIL for 72 hours were transplanted into irradiated (300 cGy) NSG mice (n = 6 each). Engraftment of human CD45+ cells in the BM of NSG mice at 4 weeks (G) and 16 weeks (H) is shown. BCR/ABL expression in the enriched human CD45+ cells in the BM at 16 weeks was measured (I). Results represent mean ± SEM. Significance values: *P < .05; **P < .01; ***P < .001, 2-way ANOVA, 1-way ANOVA. FITC-A, fluorescein isothiocyanate-A; PE-A, phycoerythrin.
To test effects on in vivo engraftment, CML and normal CD34+ cells were treated with IL-1RA, NIL, or IL-1RA plus NIL for 72 hours and transplanted into NSG mice. IL-1RA plus NIL treatment reduced short-term (4 weeks; Figure 3G) and long-term (16 weeks; Figure 3H) engraftment in BM, compared with no treatment, or IL-1RA or NIL alone. IL-1RA, NIL, and IL-1RA plus NIL all resulted in significant reduction of BCR-ABL+ cells in BM at 16 weeks, assessed by qRT-PCR analysis, compared with untreated controls (Figure 3I). The observed reduction in human cell engraftment taken together with the similar BCR-ABL levels indicates that leukemic cell engraftment is reduced with combination treatment compared with NIL alone. IL-1RA, NIL, or the combination did not significantly affect engraftment of CB CD34+ cells in PB of NSG mice at 4 weeks (supplemental Figure 2F), and in PB (supplemental Figure 2G), or BM (supplemental Figure 2H) at 16 weeks, compared with untreated controls. Taken together, these results indicate that IL-1RA when combined with NIL selectively and effectively targets primitive CML cells that resist elimination with NIL alone.
Increased IL-1 receptor expression in CML LSC
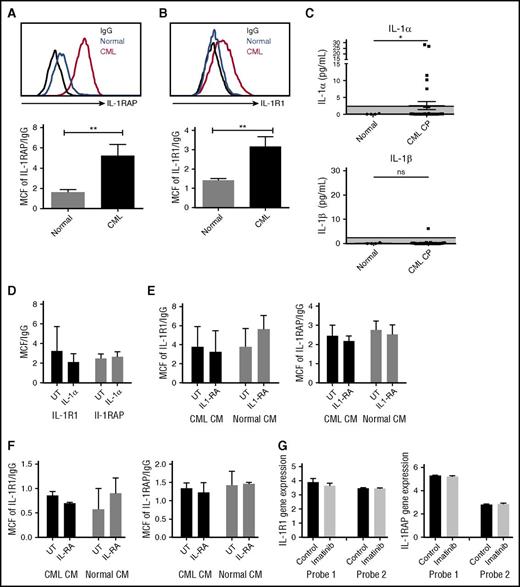
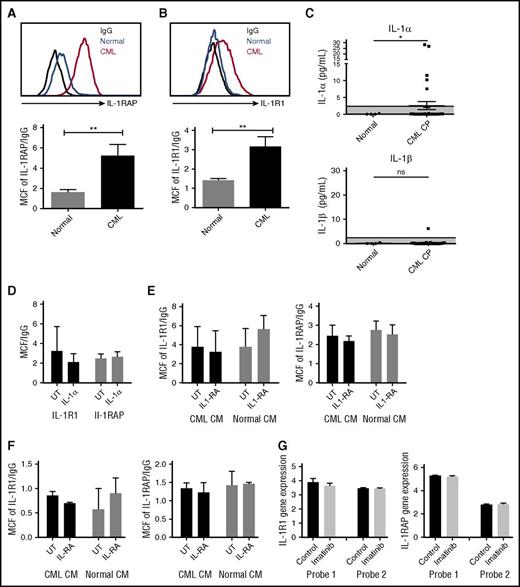
We studied expression of the IL-1 receptor components, IL-1 receptor-associated protein (IL-1RAP), and IL-1R1, on human CML and normal CD34+CD38−CD90+ stem cells and CD34+ progenitor cells (supplemental Figure 3A). Consistent with previous reports, IL-1RAP was expressed on CML but not normal stem cell populations (Figure 4A).11 We also observed a significant increase of IL-1R1 expression on CML stem cells (Figure 4B). Expression of IL-1RAP and IL-1R1 was also increased on CML compared with normal CD34+ cells (supplemental Figure 3B-C). We evaluated IL-1α and IL-1β levels in PB from CML CP patients and healthy controls by ELISA. IL-1α but not IL-1β expression was enhanced in several CML patients, and average IL-1α levels were increased compared with normal PB (Figure 4C). Exposure to IL-1α did not affect the expression of IL-1R1 and IL-1RAP on human CML stem cells (Figure 4D) and reduced IL-1RAP expression in murine CML stem cells (supplemental Figure 3D). Exposure to human CML or normal BM CM did not affect the expression of IL-1R1 and IL-1RAP on CML (Figure 4E) or normal (Figure 4F) stem cells.
Increased IL-1 receptor expression in CML LSC. IL-1RAP (A) and IL-1R1 (B) expression on human CML and normal CD34+CD38−CD90+ stem cells measured by flow cytometry. IL-1α levels in PB of CML patients and healthy controls measured by ELISA (C). Effect of human IL-1α exposure on IL-1R1 and IL-1RAP expression in CML stem cells (n = 4) (D). Effect of human CML and normal BM CM on IL-1R1 and IL-1RAP expression in CML (n = 4) (E) and normal (n = 3) (F) stem cells. IL-1R1 and IL1-RAP messenger RNA expression in CD34+ cells from CML patients after 7 days of imatinib treatment, as measured in microarray analysis, with 2 separate probes for each gene (G). Results represent mean ± SEM. Significance values: *P < .05; **P < .01, unpaired Student t test, 2-way ANOVA. MCF, median channel fluorescence.
Increased IL-1 receptor expression in CML LSC. IL-1RAP (A) and IL-1R1 (B) expression on human CML and normal CD34+CD38−CD90+ stem cells measured by flow cytometry. IL-1α levels in PB of CML patients and healthy controls measured by ELISA (C). Effect of human IL-1α exposure on IL-1R1 and IL-1RAP expression in CML stem cells (n = 4) (D). Effect of human CML and normal BM CM on IL-1R1 and IL-1RAP expression in CML (n = 4) (E) and normal (n = 3) (F) stem cells. IL-1R1 and IL1-RAP messenger RNA expression in CD34+ cells from CML patients after 7 days of imatinib treatment, as measured in microarray analysis, with 2 separate probes for each gene (G). Results represent mean ± SEM. Significance values: *P < .05; **P < .01, unpaired Student t test, 2-way ANOVA. MCF, median channel fluorescence.
We studied the effect of TKI treatment on IL-1R1 and IL1-RAP expression using data from Bruennert et al analyzing changes in the transcriptome of CD34+ cells from CML patients after 7 days of imatinib treatment.16 We observed that IL-1 receptor expression in CML progenitors was not changed after TKI treatment (Figure 4G).
IL-1RA and NIL inhibit inflammatory signaling in CML LSC
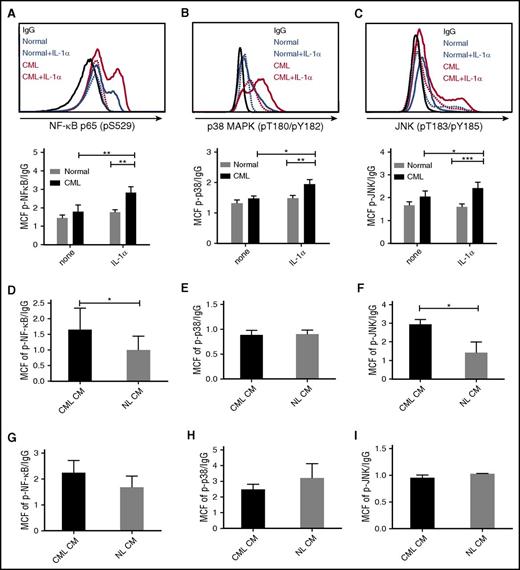
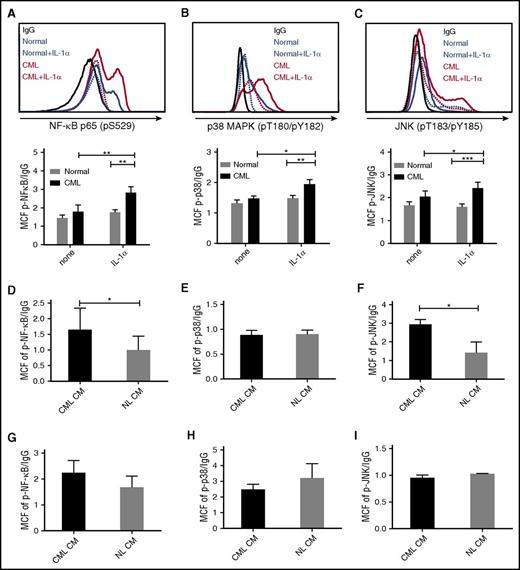
IL-1α signals through the NF-κB, p38-MAPK, and p-JNK pathways.17 Exposure to IL-1α increased expression of p-NF-κB (p65) (Figure 5A), p-p38 MAPK (Figure 5B), and p-JNK (Figure 5C) in CML, but not normal stem cells, as evaluated by flow cytometry. IL-1α also increased expression of p-NF-κB (p65) (supplemental Figure 4A) and p-p38 MAPK (supplemental Figure 4B), but not p-JNK (supplemental Figure 4C), in CML but not normal CD34+ cells. Exposure to IL-1α also increased expression of p-p38 MAPK and p-JNK in murine CML stem cells (supplemental Figure 4D). These results indicate that CML stem cells demonstrate an enhanced signaling response to IL-1 stimulation. CML stem cells demonstrated enhanced NF-κB activity (Figure 5D), no change in p-p38 activity (Figure 5E), and enhanced p-JNK activity (Figure 5F) after exposure to CML compared with normal BM CM. On the other hand, CML or normal CM did not increase NF-κB, p-p38, or p-JNK signaling in normal stem cells (Figure 5G-I).
Increased IL-1 signaling in CML LSC. CML and normal CD34+CD38−CD90+ stem cells were exposed to IL-1α (10 ng/mL) for 16 hours and expression of p-NF-κB (p65) (A), p-p38 MAPK (B), and p-JNK (C) was measured by flow cytometry. Expression of p-NF-κB (p65) (D), p-p38 MAPK (E), and p-JNK (F) in CML HSC (n = 4) exposed to human CML and normal CM for 16 hours. p-NF-κB (p65) (G), p-p38 MAPK (H), and p-JNK (I) expression in normal HSC (n = 3) exposed to CML and normal CM. Results represent mean ± SEM. Significance values: *P < .05; **P < .01; ***P < .001, 2-way ANOVA, unpaired Student t test.
Increased IL-1 signaling in CML LSC. CML and normal CD34+CD38−CD90+ stem cells were exposed to IL-1α (10 ng/mL) for 16 hours and expression of p-NF-κB (p65) (A), p-p38 MAPK (B), and p-JNK (C) was measured by flow cytometry. Expression of p-NF-κB (p65) (D), p-p38 MAPK (E), and p-JNK (F) in CML HSC (n = 4) exposed to human CML and normal CM for 16 hours. p-NF-κB (p65) (G), p-p38 MAPK (H), and p-JNK (I) expression in normal HSC (n = 3) exposed to CML and normal CM. Results represent mean ± SEM. Significance values: *P < .05; **P < .01; ***P < .001, 2-way ANOVA, unpaired Student t test.
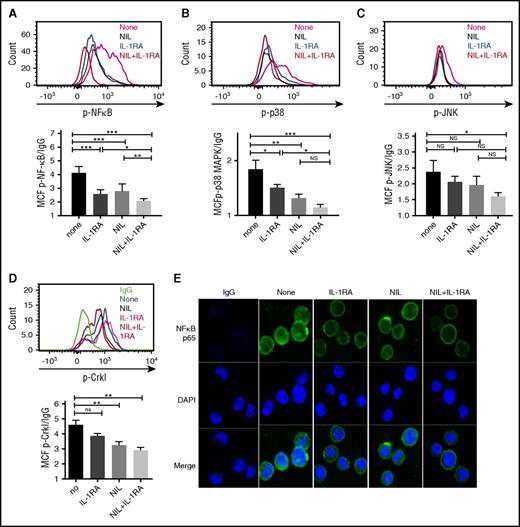
We evaluated the effect of IL-1RA, NIL, or the combination on p-NF-κB (p65), p-p38 MAPK, and p-JNK expression in CML LSC cultured in CML BM CM. Significant reduction of p-NF-κB levels was seen with IL-1RA or NIL alone, with further reduction with the combination (Figure 6A). Treatment with IL-1RA or NIL alone significantly inhibited p-p38 MAPK levels (Figure 6B), but did not inhibit p-JNK levels (Figure 6C). The combination of IL-1RA and NIL significantly reduced both p-p38 MAPK and p-JNK expression. Our results indicate that combination treatment results in additional inhibition of NF-κB signaling, but not P38 MAPK or JNK signaling compared with NIL alone (Figure 6B-C, and supplemental Figure 4F). Similar effects were seen in CML CD34+ cells (supplemental Figure 4E-G). Expression of p-CrkL was reduced in CML stem cells (Figure 6D) and CD34+ cells (supplemental Figure 4H) treated with NIL, indicating effective BCR-ABL kinase inhibition. Immunofluorescence analysis showed reduced expression of both nuclear and cytoplasmic NF-κB p65 in CML stem cells treated with IL-1RA compared with controls (Figure 6E). NIL treatment also resulted in reduction in NF-κB levels, with maximal reduction seen with the combination of IL-1RA and NIL. GSEA analysis also indicated significant inhibition of NF-kB-related genes in CD34+ cells from CML patients treated with imatinib for 7 days, compared with untreated patients (supplemental Figure 4I). qRT-PCR analysis showed that NIL treatment reduced expression of the NF-κB target genes BCL2L1 but not NFKB1A and BIRC3. On the other hand, treatment with IL-1RA and the combination of NIL and IL-1RA resulted in significant reduction in NFKB1A (Figure 7A), BCL2L1 (Figure 7B), and BIRC3 (Figure 7C), indicating additional effects of IL-1 inhibition on NF-κB signaling in CML stem cells beyond those seen with TKI treatment.
IL-1RA in combination with NIL inhibits inflammatory signaling in CML LSC. CML LTHSC (Lin-CD34+CD38−CD90+ cells) were cultured in CML BM CM in the presence of IL-1RA, NIL, or combination of IL-RA and NIL for 24 hours. The expression of p- NF-κB p65 (A), p38 MAPK (B), p-JNK (C), and p-Crkl (D) by flow cytometry is shown. Nuclear localization of NF-κB p65 protein in stem cells treated with IL-1RA, NIL, or the combination of IL-1RA and NIL was evaluated by immunofluorescence analysis (E). Results represent mean ± SEM. Significance values: *P < .05; **P < .01; ***P < .001, 1-way ANOVA. DAPI, 4′,6-diamidino-2-phenylindole.
IL-1RA in combination with NIL inhibits inflammatory signaling in CML LSC. CML LTHSC (Lin-CD34+CD38−CD90+ cells) were cultured in CML BM CM in the presence of IL-1RA, NIL, or combination of IL-RA and NIL for 24 hours. The expression of p- NF-κB p65 (A), p38 MAPK (B), p-JNK (C), and p-Crkl (D) by flow cytometry is shown. Nuclear localization of NF-κB p65 protein in stem cells treated with IL-1RA, NIL, or the combination of IL-1RA and NIL was evaluated by immunofluorescence analysis (E). Results represent mean ± SEM. Significance values: *P < .05; **P < .01; ***P < .001, 1-way ANOVA. DAPI, 4′,6-diamidino-2-phenylindole.
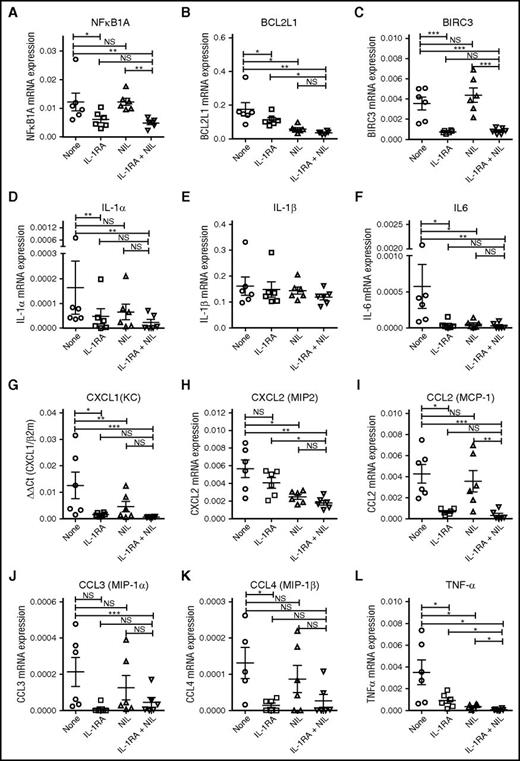
IL-1RA in combination with NIL inhibits NF-κB target gene and cytokine gene expression in CML LSC. Expression of the NF-κB target genes, NFKB1A (A), BCL2L1 (B), and BIRC3 (C) and the inflammatory cytokines, IL-1α (D), IL-1β (E), IL-6 (F), CXCL1 (G), CXCL2 (H), CCL2 (I), CCL3 (J), CCL4 (K), and TNF-α (L) in CML CD34+CD38−CD90+ cells treated with IL-1RA, NIL, or the combination of NIL and IL-1RA, was measured by qRT-PCR. Results represent mean ± SEM. Significance values: *P < .05; **P < .01, ***P < .001, paired Student t test.
IL-1RA in combination with NIL inhibits NF-κB target gene and cytokine gene expression in CML LSC. Expression of the NF-κB target genes, NFKB1A (A), BCL2L1 (B), and BIRC3 (C) and the inflammatory cytokines, IL-1α (D), IL-1β (E), IL-6 (F), CXCL1 (G), CXCL2 (H), CCL2 (I), CCL3 (J), CCL4 (K), and TNF-α (L) in CML CD34+CD38−CD90+ cells treated with IL-1RA, NIL, or the combination of NIL and IL-1RA, was measured by qRT-PCR. Results represent mean ± SEM. Significance values: *P < .05; **P < .01, ***P < .001, paired Student t test.
Because NF-κB signaling can enhance expression of several inflammatory cytokines, we evaluated the effects of IL-1RA and NIL on cytokine gene expression in CML LSC.17 qRTPCR analysis revealed reduced expression of IL-1α, but not IL-1β, with IL-1RA treatment (Figure 7D-E), and reduced expression of IL-6 (Figure 7F), CXCL1 (Figure 7G), CCL2 (Figure 7I), CCL3 (Figure 7J), CCL4 (Figure 7K), and tumor necrosis factor-α (TNF-α) (Figure 7L) with IL-1RA and IL-1RA plus NIL, compared with controls. NIL treatment reduced expression of IL-6 (Figure 7F), CXCL2 (Figure 7H), and TNF-α (Figure 7L), but not other cytokines. These observations suggest that blocking IL-1 signaling modulates expression of inflammatory mediators in CML cells.
Discussion
IL-1 receptors are expressed in HSC during fetal development and regulate their differentiation.18,19 IL1R-deleted adult mice do not show defects in steady-state hematopoiesis.20 The studies of Ågerstam et al21 confirm that IL-1RAP is dispensable for normal HSC function, even under conditions of hematopoietic stress. However, chronic IL-1 exposure can drive myeloid differentiation, and chronic exposure leads to reduced HSC self-renewal.22 Consistent with previous reports, we observed that CML LTHSC demonstrated upregulated IL-1RAP expression.11 Our studies and those of Ångerstam et al show that CML LSC also demonstrated increased expression of the coreceptor IL-1R1. Increased expression of the IL-1 receptor complex may explain enhanced IL-1 responsiveness in CML LSC. IL-1RAP is also expressed in stem cells from patients with acute myeloid leukemia, raising the possibility of similar activity in this leukemia.23,24
IL-1 is a primary regulator of inflammatory and innate immune responses. IL-1 receptor engagement initiates a complex sequence of phosphorylation and ubiquitination events resulting in activation of the NF-κB, JNK, and p38 MAPK pathways, and rapid increase in expression of a large number of immediate early genes central to the inflammatory response.17 Canonical IL-1 target genes include cytokines such as IL-6, IL-8, TNF-α, MCP-1, IL-1α, IL-1β, and MKP-1. The intracellular components that participate in IL-1 signaling also mediate responses to other cytokines. For example, autocrine TNF-α production by CML stem/progenitor cells also supports their survival by promoting NF-κB/p65 activation.25 Although other cytokines overexpressed in the CML BM microenvironment could have redundant effects, the observation that IL-1RA effectively inhibits downstream signaling through NF-κB supports a central regulatory role of IL-1. Indeed, IL-1 inhibition diminishes expression of several other cytokines in CML LSC.
IL-1 signaling is regulated at the level of synthesis and release, membrane receptors, and intracellular signal transduction. Both increased IL-1 levels in the CML BM microenvironment and increased receptor expression may play a role in enhanced signaling in CML LSC. BCR-ABL kinase–mediated modulation of intracellular signaling mechanisms could also contribute to increased IL-1 responsiveness. NF-κB is activated through the canonical IKK pathway in BCR-ABL+ cells and contributes to the pathogenesis of myeloid and lymphoid leukemia.26,27 IL-1 stimulation induces miR-146a expression, which acts as a negative feedback regulator of IL-1 signaling by inhibiting the IRAK1 and TRAF6 genes.28,29 Our recent studies show that miR-146a is downregulated in CML CD34+ cells, potentially contributing to increased IL-1 signaling.30 Further evaluation of this mechanism is warranted in future studies. The studies of Ångerstam et al, and our own analyses, show that IL-1 receptor expression on CML LSC is not reduced by BCR-ABL kinase inhibition. Ångerstam et al further show that TKI-treated CML stem cells maintain signaling responses to IL-1 stimulation. Their results are consistent with our results showing that treatment with TKI inhibits NF-κB signaling in CML LSC, but that even greater inhibition is seen after addition of IL-1RA.
Our data suggest that microenvironmental IL-1–mediated signaling contributes to resistance of CML LSC to TKI. IL-1RA had modest effects on CML stem cell maintenance by itself in the context of active BCR-ABL signaling, but when combined with NIL, resulted in significantly enhanced inhibition of CML stem cells compared with NIL alone. Currently, only a small fraction of TKI-treated CML patients can maintain TFR after discontinuing treatment.31 Our studies support further exploration of the use of IL-1 blockade as a potential strategy to enhance LSC targeting in patients receiving TKI treatment to increase the likelihood of achieving TFR. Anakinra has been extensively used to treat rheumatoid arthritis and has been well tolerated with an excellent safety profile, making it an attractive candidate for further evaluation.32 Other strategies to limit IL-1 activity approved for treating chronic inflammatory diseases include neutralizing antibodies to IL-1β and a soluble receptor for IL-1.12 Importantly, the companion paper by Ångerstam et al also shows that antibodies against IL-1RAP reduce leukemia in an in vivo model of CP CML. In conclusion, our studies reveal a role for IL-1–mediated inflammatory signaling in maintenance of CML LSC in TKI-treated CML patients and support targeting of this mechanism as a strategy to enhance LSC elimination in combination with TKI.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors acknowledge the support of the City of Hope analytical cytometry core and surgical pathology core. They thank StemCyte for CB samples, Linda Seymour and Vincent Paterno for sample acquisition, and Tinisha McDonald and Yinwei Ho for technical assistance.
This work was supported by National Institutes of Health, National Cancer Institute grant R01 CA172447, the Glasgow Experimental Cancer Medicine Centre, Bloodwise grant 14033, and Cancer Research-UK program grant C1107/A11008. V.L.C. is supported by the Wellcome Trust, the Scottish Translational Medicine, and Therapeutics Initiative (grant WT 085664) and by Clinical Research Fellowship Number 099710/X/12/Z.
Authorship
Contribution: B.Z. designed and performed experiments, analyzed and interpreted data, wrote the manuscript; S.C. designed and performed experiments, analyzed and interpreted data, wrote the manuscript; P.A. designed and performed experiments; V.L.C. designed and performed experiments; L.H. analyzed and interpreted data; H.G.J. designed and performed experiments; A.L. obtained samples, provided data; K.G. reviewed slides; T.L.H. provided samples, designed research, interpreted data, edited the manuscript; and R.B. designed research, analyzed and interpreted data, wrote the manuscript.
Conflict of interest disclosure: The authors declare no competing financial interests.
Correspondence: Ravi Bhatia, Department of Medicine, Division of Hematology-Oncology, University of Alabama at Birmingham, 1802 6th Ave South, North Pavilion, Room 2555A, Birmingham, AL 35294; e-mail: rbhatia@uabmc.edu.