In this issue of Blood, Kato et al identify compound heterozygous mutations in the Ras guanyl releasing protein 2 (RASGRP2) gene that cause decelerated platelet signaling, reduced platelet aggregation under shear stress, and severe bleeding in an adolescent patient.1
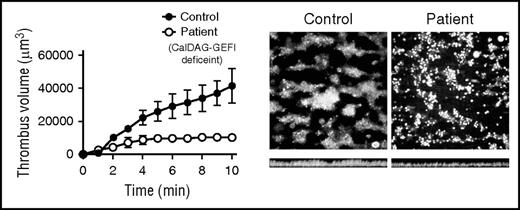
Human platelets lacking CalDAG GEFI do not form “clots” on immobilized collagen. Blood from a healthy human control or from a patient lacking CalDAG GEFI was perfused over immobilized collagen at a high shear rate intended to mimic arterial blood flow. Control platelets (left image) adhere directly to the collagen surface via multiple receptors and coalesce normally into islands of three-dimensional clotlike structures as a result of primary and secondary signaling pathways that converge upon the αIIbβ3 integrin. In contrast, the patient’s platelets (right image) adhere normally but do not form three-dimensional structures because of a lack of αIIbβ3 activation caused by the absence of CalDAG GEFI. Cross-sectional images of these adherent platelets (bottom images) demonstrate fewer platelets and smaller aggregates in the patient. Platelet numbers are clearly reduced in the patient’s clots as depicted by the plot of total fluorescence intensity (volume) on the left. See Figure 2G in the article by Kato et al that begins on page 2729.
Human platelets lacking CalDAG GEFI do not form “clots” on immobilized collagen. Blood from a healthy human control or from a patient lacking CalDAG GEFI was perfused over immobilized collagen at a high shear rate intended to mimic arterial blood flow. Control platelets (left image) adhere directly to the collagen surface via multiple receptors and coalesce normally into islands of three-dimensional clotlike structures as a result of primary and secondary signaling pathways that converge upon the αIIbβ3 integrin. In contrast, the patient’s platelets (right image) adhere normally but do not form three-dimensional structures because of a lack of αIIbβ3 activation caused by the absence of CalDAG GEFI. Cross-sectional images of these adherent platelets (bottom images) demonstrate fewer platelets and smaller aggregates in the patient. Platelet numbers are clearly reduced in the patient’s clots as depicted by the plot of total fluorescence intensity (volume) on the left. See Figure 2G in the article by Kato et al that begins on page 2729.
RASGRP2 encodes for an abundant protein in platelets known as calcium- and diacylglycerol-regulated guanine nucleotide exchange factor I (CalDAG GEFI). Much of what we know about CalDAG GEFI in the cellular context comes from the knockout mouse; disruption of the murine Rasgrp2 gene causes greatly reduced platelet signaling in response to several agonists and nearly absent thrombus formation under arterial flow conditions.2 The importance of CalDAG GEFI in the intact vasculature has also been demonstrated in several mouse models of hemostasis, atherosclerosis, and thrombotic thrombocytopenia.3-5
Even though human CalDAG GEFI is thought to perform the same function as its murine homolog, a glimpse of the physiologic role of CalDAG GEFI in human platelets has only recently been attained. A point mutation in the human RASGRP2 gene was discovered in 2 homozygous patients by whole-exome sequencing by Canault et al.6 These investigators showed that platelets expressing a CalDAG GEFI mutant (G248W) displayed decreased platelet spreading, aggregation, and adhesion under flow conditions without affecting αIIbβ3 activation or CalDAG GEFI protein levels. Even though Rap1 activation was also impaired, the G248W mutant protein is likely still capable of interacting with potential binding partners and/or effector proteins and could be acting in a dominant-negative fashion in addition to its effects on Rap1.
The present work by Kato et al extends and confirms these findings through the identification of the first true null alleles of human RASGRP2 that eliminate CalDAG GEFI protein expression in both platelets and neutrophils. Systematic phenotypic analysis of a young patient presenting with severe menstrual bleeding and a history of mucosal bleeding revealed dramatically decreased platelet aggregation in response to almost all agonists tested. Via genomic sequencing of the patient’s leukocytes, the authors discovered a nonsense mutation and a deletion mutation in exon 9 of RASGRP2, which were also found in mRNA isolated from the patient’s platelets. Importantly, separate as well as combined expression of both mutations completely prevent CalDAG GEFI protein expression in cultured cells, which provides a molecular explanation for the lack of CalDAG GEFI in the patient’s platelets and neutrophils. Unlike murine neutrophils lacking Rasgrp2, however, none of the mutations found in human RASGRP2 appear to affect neutrophil function, which may reflect yet another interesting difference between the human and murine vasculature.
Kato et al show that both Rap1 activation and activation of its downstream target αIIbβ3 are diminished and delayed in platelets lacking CalDAG GEFI. To relate this delay to a more physiologic setting, the authors directly measured the ability of the patient’s platelets to generate “clots” by perfusing the patient’s blood over immobilized collagen at high shear rates and observing clot formation by video microscopy (see figure). They found that although individual platelets lacking CalDAG GEFI adhered normally to the collagen-coated surface compared with control platelets, these platelets failed to assemble into three-dimensional clots characteristic of fully activated platelets. Furthermore, the authors previously showed that platelets from a patient lacking P2Y12, a key adenosine diphosphate (ADP) receptor that more slowly activates αIIbβ3, produce relatively normal clots compared with those formed by platelets lacking CalDAG GEFI.7 Coupled with the fact that aggregation defects in platelets lacking CalDAG GEFI are not totally rescued by high doses of agonists such as ADP, thrombin, or thromboxane mimetics, these flow chamber data strongly suggest that the primary defect in the patient’s platelets is delayed activation of αIIbβ3 rather than a lack of αIIbβ3 function or an inability to initiate secondary processes such as secretion of ADP. This concept of delayed αIIbβ3 activation leading to defective clot formation in vivo was first demonstrated in mice, but now appears to be relevant in humans.8
In summary, Kato et al describe 2 novel mutations in the human RASGRP2 gene that abolish CalDAG GEFI expression in platelets and neutrophils. The data unequivocally establish a role for CalDAG GEFI as a master regulator of multiple calcium-dependent signaling pathways leading to platelet activation, but, more importantly, provide new insight regarding the kinetics of Rap1 and αIIbβ3 integrin activation associated with clot formation and bleeding in humans. This study extends the clinical relevance of RASGRP2 but also intensifies the need to address certain questions concerning the existence of additional RASGRP2 mutations in other patients with unexplained bleeding diatheses, the prevalence of RASGRP2 mutations in these patients, and whether bleeding severity or other symptoms can be correlated with expression levels or changes in CalDAG GEFI activity. Answers to these questions will enhance our understanding of human platelet activation and likely provide new avenues for therapeutic intervention.
Conflict-of-interest disclosure: The author declares no competing financial interests.