Abstract

Background: Older patients (pts) with ALL have a worse outcome than younger pts due to poor tolerance of intensive therapy. CD22 expression is present in >90% of pts with B-cell ALL. Inotuzumab ozogamicin (InO) is a CD22 monoclonal antibody bound to a toxin, calecheamicin, and has shown single-agent activity in relapsed/refractory ALL (Kantarjian et al. NEJM 2016). Addition of targeted therapy to effective low-intensity therapy might improve outcome.
Methods: Pts ≥ 60 years with newly diagnosed B-cell ALL were eligible for the chemotherapy with mini-hyper-CVD (cyclophosphamide and dexamethasone at 50% dose reduction, no anthracycline, methotrexate at 75% dose reduction, cytarabine at 0.5 g/m2 x 4 doses) in combination with InO given on Day 3 of each of the first 4 courses. Rituximab (in patients whose cells were CD20 positive) and intrathecal chemotherapy were given for the first 4 courses. The first 6 pts received InO 1.3 mg/m2 for cycle 1 followed by 0.8 mg/m2 for subsequent cycles; Pts 7 onwards received 1.8 mg/m2 for Cycle 1 followed by 1.3 mg/m2 for subsequent cycles. After the occurrence of veno-occlusive disease (VOD) in 4 pts, the dose of InO was modified to 1.3 mg/m2 for Cycle 1 followed by 1.0 mg/m2 for subsequent cycles after Pt #33. Maintenance therapy consists of daily 6-MP and weekly methotrexate for 3 years, and monthly vincristine and 5-day prednisone for the first 1 year.
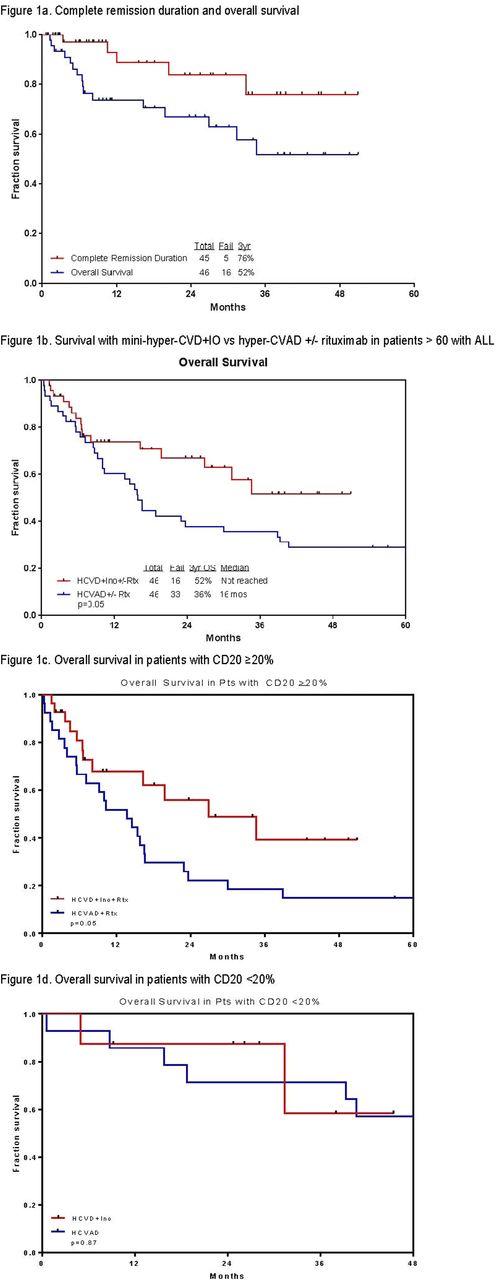
Results: Forty-six pts with a median follow-up of 24 months were treated (range 1-51 months) (Table 1). Median age is 68 years (range 60-81). Of the 42 pts evaluable for response (4 started in CR), 40 (95%) achieved complete response (CR)/ CR with incomplete platelet recovery (CRp) (35 CR, 5 CRp). All 22 pts with baseline abnormal karyotype achieved a complete cytogenetic response; of the 44 pts assessed for minimal residual disease (MRD) by 6-color multiparameter flow cytometry, 41 (93%) achieved negative MRD (71% of them at CR). Median time to platelet and neutrophil recovery was 23 days (11-91), and 16 days (0-49) after induction, and was 22 days (0-64), and 17 days (0-49) after subsequent cycles. Grade 3-4 toxicities ≥10% included prolonged thrombocytopenia (n=34; 74%), infections during consolidation (n=34; 74%), infections during induction (n=25; 54%), hyperglycemia (n=24; 52%), hypokalemia (n=16; 35%), hyperbilirubinemia (n=8; 17%), increased ALT/AST (n=9; 20%), and hemorrhage (n=6; 13%). VOD was observed in 4 pts (9%) after a median of 2 courses (2-4); all were in CR with negative MRD. Two were Grade 2 (one of them post matched related allogeneic stem cell transplantation (ASCT) using a conditioning regimen with fludarabine and busulfan after 4 cycles of mini-hyper-CVD + InO) and 2 were Grade 5. No VOD was observed after the InO dose adjustment. At the last follow-up, 30 (65%) are alive and 28 (61%) in CR. Three (7%) pts are currently receiving consolidation chemotherapy with a median of 4 cycles (1-8); 19 (41%) are receiving POMP maintenance therapy; 3 (7%) completed POMP maintenance therapy; 1 (2%) is under observation after completion of consolidation therapy. Of the 6 pts who relapsed, 2 (4%) pts are alive on salvage therapy. Three (7%) pts underwent an ASCT: 2 of them are alive in CR. Sixteen pts (35%) died: 1 had primary refractory ALL and died after the first salvage; 4 relapsed after initial response and died of disease progression; 10 (22%) died in CR from sepsis (n=4), VOD (n=2), gunshot wound (n=1), dementia (n=1), kidney dysfunction (n=1), unknown cause (n=1); and 1 died of ASCT-related complications. The 3-year complete remission duration, and overall survival rates were 76% and 52%, respectively (Figure 1a). The mini-hyper-CVD + InO +/- rituximab (n=46) results appear superior to the historical data with HCVAD +/- rituximab (n=46) in a similar patients' population (3-year overall survival (OS) rates of 52% and 36%, respectively, p=0.05); Figure 1b). This advantage was mainly observed in patients with CD20 expression ≥20% (all patients received rituximab; p=0.05) (Figure 1c); while no superiority was observed when compared to hyper-CVAD alone (in patients with CD20 expression <20%).
Conclusions: The combination of InO with low-intensity mini-hyper-CVD chemotherapy is safe and shows encouraging results in the frontline setting in older pts with ALL. These results appear to be better than those achieved with hyper-CVAD +/- rituximab and may become the new standard of care for frontline treatment of older pts with ALL.
Jabbour:ARIAD: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; Novartis: Research Funding; BMS: Consultancy. O'Brien:Pharmacyclics, LLC, an AbbVie Company: Consultancy, Honoraria, Research Funding; Janssen: Consultancy, Honoraria. Daver:Kiromic: Research Funding; Otsuka: Consultancy, Honoraria; Ariad: Research Funding; BMS: Research Funding; Pfizer: Consultancy, Research Funding; Karyopharm: Honoraria, Research Funding; Sunesis: Consultancy, Research Funding. Jain:Novimmune: Consultancy, Honoraria; Servier: Consultancy, Honoraria; Genentech: Research Funding; Novartis: Consultancy, Honoraria; BMS: Research Funding; Celgene: Research Funding; Infinity: Research Funding; Incyte: Research Funding; Pharmacyclics: Consultancy, Honoraria, Research Funding; Abbvie: Research Funding; Seattle Genetics: Research Funding; Pfizer: Consultancy, Honoraria, Research Funding; ADC Therapeutics: Consultancy, Honoraria, Research Funding. Konopleva:Calithera: Research Funding; Cellectis: Research Funding. Thompson:Pharmacyclics: Consultancy, Honoraria. Cortes:ARIAD: Consultancy, Research Funding; BMS: Consultancy, Research Funding; Novartis: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; Teva: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract