Abstract
Introduction: The Philadelphia chromosome (Ph+) is a hallmark of CML and is also present in a subset of patients with acute lymphoblastic leukemia (ALL). Lymphoid blast phase (CML-BP) and Ph+ ALL almost always affect B cell lineage. In this report, we present the clinical characteristics of the rare subset of patients with Ph+ ALL or CML-BP with T-cell lymphoid phenotype.
Methods: We reviewed the institutional database for all patients with blast phase CML (n=498) and de novo T-ALL (n=150) diagnosed since 07/1997. All clinical characteristics at the time of the diagnosis of BP or Ph+ ALL were collected. Seven patients with Ph+ T-cell lymphoid leukemia were identified.
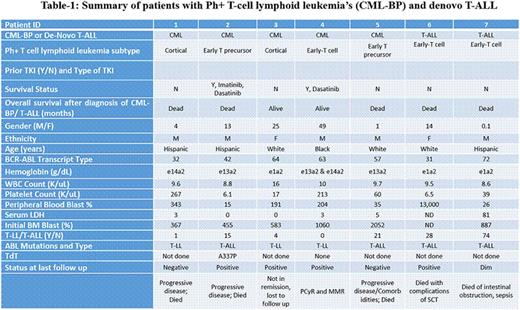
Results: Among the total of 498 CML-BP patients, 5 patients had T-cell lymphoid CML-BP (0.01%); in addition 2 patients with de novo Ph+ T-ALL were identified among 150 patients with T-ALL seen during the same time period (1.3%). Median age of the seven patients was 57 years (range 31-72 years); 71% were male. Among patients with CML-BP, all were in BP at the time they were referred to MDACC (one had progressed from an initial diagnosis in CP, 1 from accelerated phase, and 3 were in BP at initial diagnosis). Only 2 patients with CML-BP received prior TKI therapy; the 2 patients with T-ALL were previously untreated at the time of referral. Immunophenotype included 2 cortical, 3 early T-cell and 2 early T-cell precursor; 2 patients were negative and one was dim positive for TdT (summarized in Table-1). Extramedullary disease at initial presentation was seen in all patients [2 with lymphadenopathy alone, 2 with mediastinal involvement (one also with lymphadenopathy and the other with splenomegaly and lymphadenopathy), 2 with pleural effusion (1 with lymphadenopathy and another with splenomegaly) and 1 patient with splenomegaly alone]. None had CNS involvement at initial presentation, but one patient developed CNS involvement at first relapse. One patient had variant Ph+ and 2 had complex karyotype. Three patients had lymphoblastic lymphoma and 4 patients had T-ALL. Five patients died (3 from disease progression, one from complications of SCT, and one from intestinal obstruction and sepsis) and 2 are alive (25 and 49 months from BP diagnosis). The median survival for all patients was 13 months (range 1-49 months).
Five patients received hyper-CVAD based therapy as an induction regimen: 3 of them received hyper-CVAD alone - one achieved CR then underwent a SCT but died due to complications of SCT, another achieved PR and was given imatinib followed by dasatinib which he did not tolerate; he was subsequently lost to follow up; and one patient had no response. The other 2 patients received hyper-CVAD with dasatinib - one achieved PR, then underwent allo-SCT and achieved CR but later had a CNS relapse and progressed; the other patient developed nodal progression 9 months after achieving CR with HCVAD-dasatinib which was later found to represent metastatic prostate cancer in the lymph nodes with no T-ALL recurrence and is currently on dasatinib with major molecular response. Two patients never received any TKI therapy and progressed on chemotherapy alone. Two patients received nelarabine as first salvage - one as a single agent (no response) and another with dasatinib (with PR).
Conclusions: Ph+ T-cell lymphoid leukemias are very rare and have clinical features similar to those of de novo T-ALL. Induction with a combination of HCVAD and TKIs may achieve prolonged remission in some patients.
Jabbour:ARIAD: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; Novartis: Research Funding; BMS: Consultancy. O'Brien:Pharmacyclics, LLC, an AbbVie Company: Consultancy, Honoraria, Research Funding; Janssen: Consultancy, Honoraria. Cortes:ARIAD: Consultancy, Research Funding; BMS: Consultancy, Research Funding; Novartis: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; Teva: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.