Abstract
Objectives.Bosutinib is a second-generation tyrosine kinase inhibitor (TKI) indicated for patients with previously treated Philadelphia chromosome positive chronic myeloid leukaemia (CML) when imatinib, nilotinib and dasatinib are not appropriate. Data describing the use of bosutinib in patients with CML in the real world clinical setting are limited. The objective of this study was to describe the efficacy and safety of bosutinib in patients with CML used under routine clinical practice.
Methods.An international, multi-centre, retrospective, non-interventional study in hospitals in the UK (n=7) and the Netherlands (n=2). Fifty-three patients (32 [60%] male) with CML, aged ≥18 years at bosutinib initiation and with ≥3 months data available post-initiation were recruited. Surviving patients provided written informed consent for data collection; data from deceased patients were collected by a member of the direct care team to preserve confidentiality. Data were analysed using descriptive statistics with no imputation of missing values (denominators presented where data are missing).
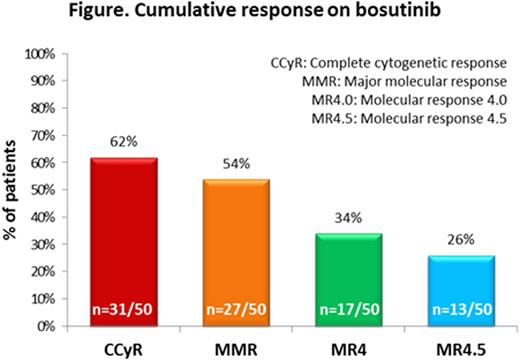
Results.Median age at bosutinib initiation was 63.6 (range: 25.5 to 90.1) years; median time from CML diagnosis to bosutinib initiation was 7.1 (range: 0.5 to 35.7) years; 74% (39/53) of patients had one or more co-morbidities at initiation. Bosutinib was 3rd-line TKI in 32% (17/53) of patients and 4th-line TKI in 53% (28/53) of patients. Fifty-seven percent (30/53) of patients switched to bosutinib due to intolerance and 26% (14/53) due to resistance to a previous TKI. The most common bosutinib starting dose was 300 mg/day (28% [15/53] of patients). Median bosutinib treatment duration was 15.6 (range: 0.4 to 66.0) months. The proportions of patients with cumulative complete cytogenetic response (CCyR), major molecular response (MMR), molecular response 4.0 (MR4.0) and molecular response 4.5 (MR4.5) with bosutinib were 62%, 54%, 34% and 26%, respectively (see figure). At data collection (median follow-up 25.1 [range: 3.0 to 66.0] months), 38% (20/53) of patients had discontinued bosutinib; 2% (1/53) discontinued due to progression, 8% (4/53) due to treatment failure, 15% (8/53) due to adverse events (AE), 6% (3/53) due to loss of response, and 4% (2/53) due to patient request. Ninety-two percent (49/53) of patients experienced ≥1 AE, most commonly diarrhoea (55% [29/53] of patients); 26% (14/53) of patients had grade 3/4 AEs (diarrhoea grade 3/4: 4% [2/53] of patients).
Conclusions.Bosutinib used in normal clinical practice in pre-treated patients with CML demonstrates high rates of cytogenetic and major molecular response similar to those seen in clinical trials. Bosutinib is generally well tolerated, with the majority of patients not experiencing grade 3/4 AEs, despite most patients having pre-existing co-morbidities.
Apperley:Pfizer: Honoraria, Speakers Bureau; Ariad: Honoraria, Speakers Bureau; Bristol Myers Squibb: Honoraria, Speakers Bureau; Incyte: Speakers Bureau; Novartis: Honoraria, Speakers Bureau. Byrne:Bristol Myers Squipp: Consultancy, Speakers Bureau. Smith:Pfizer: Honoraria, Other: Advisory boards and talks at regional sponsored meetings. Viqueira:Pfizer: Employment. Ferdinand:Pfizer: Employment. Carter:pH Associates: Employment. Nock:pH Associates: Employment. Milojkovic:Ariad: Honoraria; Pfizer: Honoraria; Novartis: Honoraria; Bristol Myers Squibb: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal