Abstract
The focus of ATO resistance in acute promyelocytic leukemia (APL) has centered on mutations in PML-RARA gene (Blood 2011, NEJM 2014). However such mutations are rare and cannot explain the majority of relapses seen in the clinic. To evaluate the mechanisms of ATO resistance, we generated ATO resistant NB4 sub clone NB4-EVAsR1 (A216V - VAF-91.7%) in our laboratory. We also had another ATO resistant cell line (UF1) which does not have the A216V mutation. In an expression array we noted that redox signaling, AMPK signaling and energy metabolism pathways were significantly dysregulated in the ATO resistant cell lines compared to naïve NB4 cells.
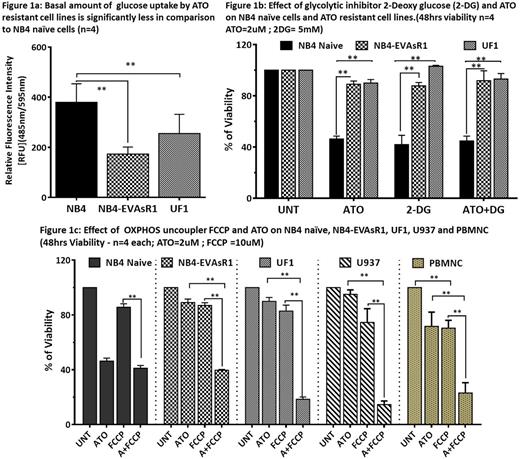
Towards validating the microarray data and to characterize the ATO resistant cell lines we measured the basal levels of reactive oxygen species (ROS), glutathione(GSH), mitochondrial membrane potential (MMP), glucose uptake and their sensitivity to glycolytic inhibitor 2-Deoxy glucose (2-DG) in comparison to naïve NB4 cells. We observed that resistant cell lines have significantly lower ROS, MMP, glucose uptake (Fig 1a) and increased GSH. We also observed that the resistant cell lines were significantly less susceptible to treatment with 2-DG in comparison to naïve NB4 cells (Fig 1b) suggesting that resistant cell lines were less dependent on glycolysis. ATO has been reported to directly inhibit the glycolytic pathway, this effect is believed to contribute to its cytotoxic effect (PNAS 2015). However, we did not observe any cytotoxic synergy between ATO and 2-DG on naïve NB4 cells and neither did this combination restore sensitivity to ATO in the resistant cell lines (Fig 1b).
Next we assessed the sensitivity of these resistant cell lines to oxidative phosphorylation (OXPHOS) inhibitors. We used an uncoupler (FCCP at 10uM) of OXPHOS which promotes uncoupled respiration by deregulating the proton gradient which drives ATP synthesis via ATP synthase. We observed that the FCCP treatment alone did not reduced the viability of naïve NB4 cells. Similarly, viability of ATO resistant cell lines also did not reduce significantly suggesting the ability of these cells to uncouple their metabolic pathway from OXPHOS to glycolysis when inhibited. However, when FCCP was combined with ATO it significantly restored the sensitivity of the resistant cell lines to ATO (Fig 1c). The same combination did not have any additive effect on naïve NB4 cells. The combination not only restored the sensitivity of the ATO resistant cell lines but also sensitized the conventionally ATO resistant cell lines such U937 (Fig 1c) and THP1. In spite of the profound effect on leukemic cells we also observed a significant bystander effect on the normal peripheral blood mononuclear cells (Fig 1c). The data suggests that the sensitivity of these resistant cell lines could be potentially restored by combining ATO with an OXPHOS uncoupler. A number of molecules that are FDA approved and used in the clinic also have OXPHOS uncoupling activity and could potentially be evaluated for their synergistic activity with ATO in leukemia. This data also draws attention to possible severe systemic off-target toxicity of such combinations which may be inadvertently used in the clinic.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.