Abstract
Introduction
Treatment-resistant acute lymphoblastic leukemia (ALL) remains a significant clinical issue. Recently, genomic profiling has identified a new subtype of high-risk ALL termed Philadelphia-chromosome-like (Ph-like) ALL, associated with a poor outcome1. Ph-like ALL has a gene expression profile similar to Ph+ (BCR-ABL1+) ALL, characterized by the presence of fusion genes converging on kinase and cytokine signaling pathways. These pathways have been shown to be targetable both in vitro and in case reports by tyrosine kinase inhibitors (TKIs). Despite well-documented efficacy profiles, it is known from TKI-use in chronic myeloid leukemia (CML) andPh+ ALL that resistance is likely, resulting in relapse. Our study aims to model and understand mechanisms of TKI-resistance inPh-like ALL, informing future therapeutic strategies that may avert or overcome resistance, potentially improving patient outcomes.
Methods
Three Ph-like ALL lines were generated via retroviral-transduction from plasmids of fusion genes identified in patient cohorts (RANBP2-ABL1, SSBP2-CSF1R and PAX5-JAK2, a kind gift from C. Mullighan)2 into Ba/F3 pro-B cells. Transformation was confirmed via growth of cells in the absence of IL-3. Cells were tested for sensitivity to a panel of TKIs (imatinib, dasatinib, ponatinib, ruxolitinib and BMS-911543) via Annexin-V/7-AAD flow-cytometry and western blotting of downstream effector proteins. Drug resistance was generated through exposure of cells to incrementally increasing concentrations of TKIs over a period of 3-6 months, and cell death LD50 determined byAnnexin-V/7-AAD. Sanger sequencing of the 3-prime partner gene of each fusion was performed to identify the emergence of any kinase-domain mutations.
Results
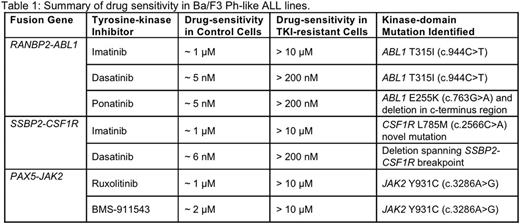
Ba/F3 Ph-like cells demonstrated sensitivity to TKIs at clinically relevant doses (RANBP2-ABL1: 1 μM imatinib, 5 nM dasatinib & 5 nM ponatinib; SSBP2-CSF1R: 1 μM imatinib, 6 nM dasatinib; PAX5-JAK2: 1 μM ruxolitinib & 2 μM BMS-911543). This correlated with decreased levels of relevant downstream signaling proteins including p-Stat5, p-Erk and p-CrkL. TKI-resistant Ph-like ALL lines were tolerant to a significantly higher concentration of TKIs compared to control (RANBP2-ABL1: 10 μM imatinib, 200 nM dasatinib & 200 nM ponatinib; SSBP2-CSF1R: 10 μM imatinib, 200 nM dasatinib; PAX5-JAK2: 10μMruxolitinib & 10μM BMS-911543; Table 1).
Sequencing analysis revealed that Ba/F3 RANBP2-ABL1 imatinib and dasatinib resistant cells acquired the clinically significant ABL1 T315I (c.944C>T) kinase-domain mutation, which was ultimately targetable using the third-generation TKI ponatinib (LD50: 25 nM). An ABL1 E255K (c.763G>A) and c-terminus deletion was discovered in the ponatinib-resistant line. In Ba/F3 SSBP2-CSF1R cells, a novel CSF1R L785M (c.2566C>A) mutation was identified in imatinib-resistant cells whereas a deletion spanning the SSBP2-CSF1R breakpoint was acquired in the dasatinib-resistant line. A JAK2 Y931C (c.3286A>G) point mutation previously associated with resistance to ATP-competitive inhibitors was acquired in Ba/F3 PAX5-JAK2ruxolitinib and BMS-911543 resistant lines.
Conclusion
In vitro modeling of Ph-like ALL resistance has identified novel kinase domain mutations and deletions that may arise as a result of targeted TKI therapy. In addition, previously identified mutations (T315I and E255K) were also identified. Detection of these mutations is important because alterations in drug-binding regions are known to result in significantly reduced TKI sensitivity, leading clinically to relapse3. This study describes an in vitro platform that can be utilized to inform future clinical approachesincluding the development of rational therapeutic approaches (and/or combination therapies) to avert resistance inPh-like ALL cases treated with rationally targeted therapies.
Abbreviations:
ABL1 - Abelson tyrosine protein kinase 1
CSF1R - Colony stimulating factor 1 receptor
JAK2 - Janus kinase 2
PAX5 - Paired box 5
RANBP2 - RAN-binding protein 2
SSBP2 - Single-stranded DNA binding protein 2
References:
1 Den Boer et al, Lancet Oncology 2009; 10(2):125-34
2 Roberts et al, Cancer Cell 2012; 22(2):153-66
3 Barouch-Bentov & Sauer, Expert Opinion on Investigational Drugs 2011; 20(2);153-208
Hughes:Bristol-Myers Squibb: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis Pharmaceuticals: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Ariad: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Australasian Leukaemia and Lymphoma Group (ALLG): Other: Chair of the CML/MPN Disease Group. White:Bristol-Myers Squibb: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis Pharmaceuticals: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Ariad: Consultancy, Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal