In this issue of Blood, the first prospective trial of unrelated donor bone marrow transplantation (BMT) in children with sickle cell disease (SCD), reported by Shenoy et al, is an important step in extending curative therapy to more children with severe disease.1
Outcome after unrelated donor BMT for children with SCD: results of the phase 2 SCURT trial. See Figures 1 and 2 in the article by Shenoy et al that begins on page 2561.
Outcome after unrelated donor BMT for children with SCD: results of the phase 2 SCURT trial. See Figures 1 and 2 in the article by Shenoy et al that begins on page 2561.
Matched related donor BMT for SCD is now well established as a treatment option for children with severe disease.2,3 Overall, the outcome is extremely good, and BMT remains the best immediate prospect for long-term cure for children who are currently experiencing severe sickle-related complications.2,3 Compared with other disease-modifying therapies, such as regular transfusion and hydroxycarbamide, matched related donor BMT will not only stop ongoing vaso-occlusive crises and arrest progressive organ damage in most cases, but may also lead to regression of damage in some cases.3 Aggregate results from case series and clinical trials show disease-free survival of ∼92% and overall survival (OS) of ∼95% of children and young adults with SCD with a matched sibling donor. Crucially, <1 in 10 of these families will have to cope with the major adverse outcome of BMT transplant-related mortality (TRM) and severe chronic graft-versus-host disease (GVHD).2,3 Given that SCD causes premature death and disability, particularly in young adults, and has a major impact on the quality of life for patients at all ages and their families,4 these are convincing results.
Can similarly impressive results be achieved for the 80% to 90% of patients with SCD who do not have HLA-matched family donors? A handful of small studies, using haplo-identical, cord blood (CB), or CD34+ peripheral blood cells from matched unrelated donors to treat patients with severe SCD, report an OS of 75% to 100%, although this is at the cost of high rates of graft rejection (38-60%).3
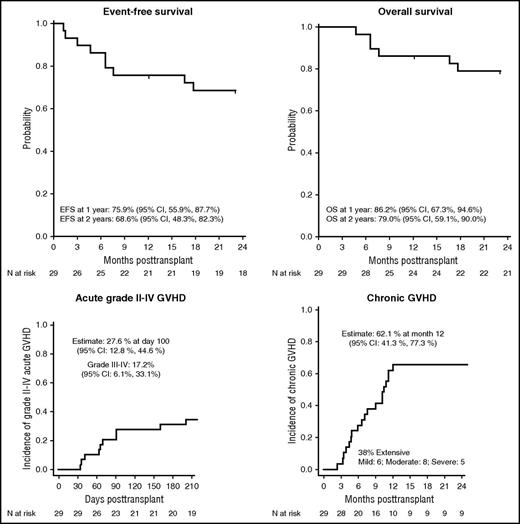
The Blood and Marrow Transplant Clinical Trials Network Sickle Cell Unrelated Donor Transplant (SCURT) study (BMTCTN 0601; #NCT00745420)1 aimed to improve on these results by using a reduced-intensity conditioning (RIC) regimen. The study was conducted between 2008 and 2014 and originally had 2 arms: the BMT arm, the results of which are presented here,1 and a CB transplant arm, which closed early due to a high rejection rate and has been reported separately.5 Twenty-nine children (age, 3-19 years) were treated in the RIC BMT arm of the study using a regimen consisting of alemtuzumab, fludarabine, and melphalan; GVHD prophylaxis was cyclosporine or tacrolimus together with short-course methotrexate and methylprednisolone. In contrast to a similar study in adults,6 no irradiation was used. Nevertheless, the graft rejection rate was low (10%): 27 of 29 patients engrafted, and 1 patient experienced secondary graft rejection. The 1-year and 2-year event-free survival was 76% and 69%, respectively, with an OS of 86% and 79% (see figure). GVHD was the principal transplant-related complication: the day 100 incidence of grade II to IV acute GVHD was 28%, and the 1-year incidence of chronic GVHD was 62% (see figure), which was extensive in 38% of the transplanted children. Overall, 8 of 29 children (27.6%) died, 7 of these as a result of complications of acute or chronic GVHD.
For pediatric hematologists, the results of the SCURT study are disappointing; for some of the families, a personal tragedy. Nevertheless, this is an extremely important study. Not only is this the first prospective trial of unrelated donor transplantation in SCD, but it involved multiple centers and has been steered through with the support of an independent external review committee to reach completion, allowing the results to be shared and lessons to be learned. In particular, as the investigators highlight, this specific RIC protocol was associated with an unacceptably high TRM, mainly due to GVHD. Although the reasons for this are difficult to assess here without full details of each individual case, possible factors may include the timing of the alemtuzumab (early in the protocol) and toxicity in this population of the combination of several agents with gut toxicity. Hence, the approach that enabled a high rate of engraftment in a RIC setting may have been responsible for the high TRM. Advances in the prevention of GVHD associated with haploidentical transplantation may offer a way forward.2
The outcome of the SCURT study inevitably raises questions about the role of alternative donor stem cell transplantation in the management of children with SCD. Although the inclusion criteria for SCURT permitted unrelated donor BMT as an alternative to conventional disease-modifying treatment of recurrent vaso-occlusive crises or ≥2 episodes of acute chest syndrome in the preceding 2 years, for most such children, hydroxycarbamide is effective and well tolerated. Similarly, many SCD children with central nervous system (CNS) disease will do very well on regular transfusions. However, there remains a group of severely affected SCD children who fail hydroxycarbamide or who have a very high rate of CNS disease progression despite receiving chronic blood transfusion therapy.7 The majority of these children will not have an HLA-matched sibling donor. It is for these children that well-designed protocols for alternative donor transplantation are urgently needed. Although the SCURT trial confirms the feasibility of unrelated transplantation in SCD, it also shows that significant adjustments to the protocol will be needed to combat the high rate of severe GVHD. This is important to tackle, as experience following transplantation for thalassemia shows that chronic GVHD is the largest single factor preventing a normal quality of life.8
The decision to offer transplantation, particularly from an alternative donor, as a treatment option to families of children with SCD is always very difficult. Application of a precision medicine approach to improve prognosis prediction in SCD would be a major advance. In the meantime, although gene therapy is an attractive future prospect9 for children who are currently severely affected and failing conventional therapy, allogeneic transplantation is still the best realistic option of cure and of arresting progressive organ damage. SCURT highlights the importance of using new protocols in a clinical trial setting, despite the well-recognized difficulties of low recruitment and completion of studies in SCD.10
Conflict-of-interest disclosure: The authors declare no competing financial interests.