Abstract
Uniformly adopted response criteria are essential for assessment of therapies incorporating conventional chemotherapy and chemoimmunotherapy regimens. Recently, immunomodulatory agents, such as immune checkpoint inhibitors, have demonstrated impressive activity in a broad range of lymphoma histologies. However, these agents may be associated with clinical and imaging findings during treatment suggestive of progressive disease (PD) despite evidence of clinical benefit (eg, tumor flare or pseudo-progression). Considering this finding as PD could lead to patients being prematurely removed from a treatment from which they actually stand to benefit. This phenomenon has been well described with checkpoint blockade therapy in solid tumors and anecdotally seen in lymphoma as well. To address this issue in the context of lymphoma immunomodulatory therapy, a workshop was convened to provide provisional recommendations to modify current response criteria in patients receiving these and future agents in clinical trials. The term “indeterminate response” was introduced to identify such lesions until confirmed as flare/pseudo-progression or true PD by either biopsy or subsequent imaging.
History of response criteria for lymphoma with conventional therapy
The first universally accepted response criteria for non-Hodgkin lymphoma (NHL) were published in 1999 by an International Working Group and were also adopted for Hodgkin lymphoma (HL).1 These International Working Group guidelines defined complete remission (CR), partial remission (PR), complete remission unconfirmed, stable disease (SD), relapsed disease, and progressive disease (PD), based on physical examination, chest x-ray, computed tomography (CT) scan, single photon emission computed tomography gallium scans, and visual bone marrow evaluation. The subsequent availability of positron emission tomography (PET), as well as immunohistochemistry and flow cytometry of the bone marrow, resulted in the revised 2007 guidelines, including PET as a component of response assessment primarily for HL and diffuse large B-cell lymphoma (DLBCL).2 At the time, PET was recommended for posttreatment assessment of DLBCL and HL, but only in clinical trials for follicular lymphoma or other fluorodeoxyglucose (FDG) avid histologies. Further experience with the interpretation of PET scan results subsequently led to the Lugano Classification for staging and response assessment,3 incorporating PET-CT as a standard component of both the staging and response assessment of FDG-avid histologies while retaining CT evaluation for other subtypes.
Response patterns with nonconventional therapies
As with prior criteria, the Lugano Classification was based on experience with traditional chemotherapeutic or chemoimmunotherapeutic regimens, primarily incorporating rituximab. However, the availability of an increasing number of biologic agents with immune mechanisms entering the clinic requires flexibility in interpretation of the recommendations to account for these agents’ biologic or immunomodulatory properties.
Tumor flare was first formally described with immunomodulatory drugs, particularly lenalidomide, in patients with lymphomas and chronic lymphocytic leukemia (CLL).4-7 In ∼15% of patients with CLL/small lymphocytic lymphoma (SLL), and less commonly in other lymphoma histologies, a “tumor flare” occurs, generally during the first 2 to 3 weeks of treatment. This phenomenon is characterized by a rapid, often painful, self-limited increase in the size of lymph nodes and is often accompanied by fever, lymphocytosis, rash, and bone pain.8,9 The pathophysiology is speculated to be related in part to an immune phenomenon characterized by natural killer cell activation, modulation of costimulatory (CD80, CD83, CD86) surface molecules on CLL cells in vitro and in vivo, and an increase in levels of tumor necrosis factor-α post-lenalidomide treatment, consistent with an acute inflammatory reaction.10 Strict application of currently used CLL11,12 or lymphoma guidelines3 to patients receiving immunomodulatory drugs could result in incorrect assignment of PD, resulting in early cessation of therapy prior to achieving clinical benefit. Other drugs with reported flare reactions include rituximab,13,14 which may also cause a paradoxical increase in immunoglobulin M with an increase in viscosity in patients with Waldenström macrglobulinemia,15,16 and brentuximab vedotin.17
Another instance of atypical response pattern occurs with inhibitors of B-cell receptor signaling pathways. Bruton tyrosine kinase and phosphatidylinositol 3-kinase targeting agents are associated with major activity in CLL/SLL, mantle cell, and other lymphomas, and are altering treatment paradigms.18,19 In patients with CLL/SLL, and less often in other lymphomas, both idelalisib and ibrutinib may cause a rapid reduction in lymph node size and spleen mass, often with improvement of cytopenias, but associated with lymphocytosis.20-22 This finding, which relates to a redistribution of lymphocytes from tissue sites to the peripheral blood,23,24 may persist for a year or longer without signs or symptoms associated with disease progression and does not represent a suboptimal response to therapy. Rigorous application of the National Cancer Institute Working Group criteria of 1996 or the International Workshop on Chronic Lymphocytic Leukemia of 2008 would result in the incorrect designation of PD in a significant number of patients, leading to premature discontinuation of drug despite other evidence of clinical improvement. Over time, many of these responses improve to partial or even complete responses as the lymphocytosis resolves. This entity is now referred to as partial response with lymphocytosis,25 which more accurately reflects the favorable nature of the response. Thus, the focus on traditional overall response rates is misleading and underestimates the magnitude of the clinical benefit of these agents.
Response assessment with immune checkpoint inhibitors
The newest agents with which atypical responses are encountered are the immune checkpoint inhibitors. These drugs counter the tumor’s usurpation of normal costimulatory or coinhibitory immune regulatory pathways, thereby reactivating endogenous tumoricidal immune activity. In solid tumors, anticytotoxic T lymphocyte–associated-4 monoclonal antibodies (eg, ipilimumab), and anti–programmed cell death-1 monoclonal antibodies (eg, nivolumab, pembrolizumab) have achieved impressive results in patients with lung cancer, melanoma, renal cell carcinoma, and other tumor types.26-29 Nevertheless, response assessment may be confounded by a delayed effect of the drugs, allowing early tumor growth, or by therapeutic immune activation manifesting as an increase in the size of existing lesions or even the appearance of new lesions, so-called delayed response or pseudo-progression. The recognition of these phenomena triggered efforts to formally characterize them and to modify standard response criteria to account for them.30 For example, with ipilimumab monotherapy, 4 distinct response patterns have been reported: (1) shrinkage in baseline lesions, without new lesions; (2) durable stable disease (in some patients followed by a slow, steady decline in total tumor burden); (3) response after an increase in total tumor burden; and (4) response in the presence of new lesions. All patterns have been associated with survival that is similar to those with typical responses.31 To account for these phenomena, Wolchok et al31 proposed immune-related response criteria (IRC) that have been incorporated into current trials of checkpoint blockade in patients with solid tumors (Table 1). The core concepts of IRC are as follows30 :
Confirmation of progression via a subsequent scan to detect delayed responses (time point to be chosen based on characteristics of the disease under study);
Measuring new lesions to include them into the total tumor volume;
Accounting for durable stable disease as benefit; and
Treating beyond conventional progression if the clinical situation allows.
IRC for solid tumors
| Response designation . | Definition . |
|---|---|
| ir complete remission | Complete disappearance of all lesions (whether measureable or not, and no new lesions), confirmed by a repeat, consecutive assessment no less than 4 weeks from the date first documented |
| ir partial remission | Decrease in tumor burden ≥50% relative to baseline confirmed by a consecutive assessment at least 4 weeks after first documentation |
| ir stable disease | Not meeting criteria for irCR or irPR, in absence of irPD |
| ir progressive disease | Increase in tumor burden ≥25% relative to nadir (minimum recorded tumor burden), confirmed by a repeat, consecutive assessment no less than 4 weeks from the date first documented |
| Response designation . | Definition . |
|---|---|
| ir complete remission | Complete disappearance of all lesions (whether measureable or not, and no new lesions), confirmed by a repeat, consecutive assessment no less than 4 weeks from the date first documented |
| ir partial remission | Decrease in tumor burden ≥50% relative to baseline confirmed by a consecutive assessment at least 4 weeks after first documentation |
| ir stable disease | Not meeting criteria for irCR or irPR, in absence of irPD |
| ir progressive disease | Increase in tumor burden ≥25% relative to nadir (minimum recorded tumor burden), confirmed by a repeat, consecutive assessment no less than 4 weeks from the date first documented |
Adapted from Wolchok et al.31 ir, immune response.
Recently, Hodi et al32 compared the predictability of overall survival and best overall response in a study of 655 patients with melanoma treated with pembrolizumab and showed that 5% had early pseudo-progression, whereas 3% experienced delayed pseudo-progression. They concluded that, using Response Evaluation Criteria In Solid Tumor (RECIST), response would have been underestimated in 15% of patients, potentially resulting in premature termination of effective therapy.
Checkpoint inhibitors have also demonstrated impressive activity in HL,33 as well as activity across various other subtypes of NHL.34,35 Not surprisingly, flare reactions or delayed responses similar to those in patients with solid tumors have been observed on those trials. These atypical responses are characterized either by the early progression of existing lesions, later followed by response, or by the development of new lesions, with or without tumor shrinkage elsewhere.
At the present time, it is unclear how these reactions can be reliably identified and distinguished from true disease progression, as the breadth of experience accumulated in solid tumors is lacking in lymphoma. To maximize both our understanding of these responses and the potential benefit of these therapies in patients with lymphoma, there is an urgent need for a framework to optimally categorize, report, and manage these atypical responses. Such criteria are critical for treating physicians to optimally use checkpoint inhibitor therapy, in order that effective therapy is not discontinued prematurely in patients experiencing benefit, as would occur using conventional response criteria. In addition, it is important to gain experience in treating patients past the occurrence of conventional PD and to formally collect and analyze this information, to ascertain whether or not treatment past PD in formally defined circumstances can indeed provide a clinical benefit.
The concepts that underlie the IRC can also be applied to patient with lymphoma and would clearly alter response assessment in some cases (Figures 1 and 2). Yet simply applying the IRC criteria derived from patients with solid tumors to lymphoma may not be adequate (Figures 1 and 2). First, lymphoma response is currently assessed per the Lugano Classification3,36 rather than RECIST (which is the basis for the IRC). This reflects the separate evolution of response criteria specifically designed for patients with lymphoma, as described above. Second, the IRC recommend restaging with a confirmatory study no less than 4 weeks following the initial assessment for aggressive tumors such as melanoma. In patients with lymphoma treated with a finite number of cycles of treatment, restaging is not typically recommended until 6 to 8 weeks following completion of therapy to minimize false-positive results. Third, confirmatory studies are not required in lymphoma, because a response that does not persist for >8 weeks is not usually considered clinically meaningful. Fourth, the IRC require a ≥25% increase in the bi-dimensional World Health Organization (WHO) criteria for solid tumors of a single lesion for PD, whereas an increase in the size of a single node is sufficient for considering lymphoma progression under certain circumstances.3 Last, the IRCs are based on assessing tumor masses, which are always abnormal. By contrast, lymph nodes, which are normally present, may be normal in size despite involvement by lymphoma, or may be enlarged by benign processes (eg, fibrous or inflammatory tissue).
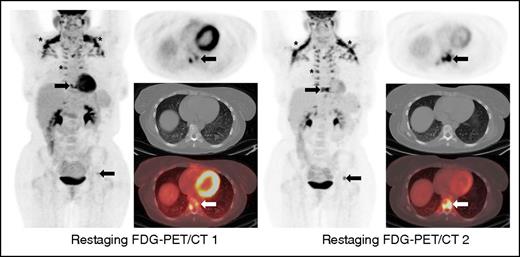
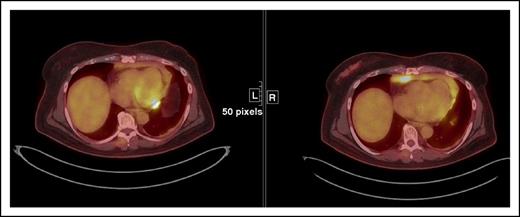
This case illustrates a discrepancy between the revised Lugano Classification (PD) and the immune-related response criteria (PR) given the fact that the immune-related response criteria do not take into consideration PET/CT findings. This type of discrepancy is particularly notable in cases with bone marrow involvement. Oftentimes, lymphomatous involvement of the bone marrow is either not measurable (due to absence of soft tissue component) or imperceptible on CT. Therefore, these findings cannot be integrated in the tumor burden of the immune-related response criteria. Restaging PET-CT is at 12 weeks. Restaging PET/CT 2 at 20 weeks demonstrates new areas of FDG uptake in the left side of T9 vertebral body (arrows) and increasing uptake in the left acetabulum, suggesting increasing extent of marrow disease, whereas this is barely seen on CT. Marked physiologic uptake is also seen in brown fat (asterisks).
This case illustrates a discrepancy between the revised Lugano Classification (PD) and the immune-related response criteria (PR) given the fact that the immune-related response criteria do not take into consideration PET/CT findings. This type of discrepancy is particularly notable in cases with bone marrow involvement. Oftentimes, lymphomatous involvement of the bone marrow is either not measurable (due to absence of soft tissue component) or imperceptible on CT. Therefore, these findings cannot be integrated in the tumor burden of the immune-related response criteria. Restaging PET-CT is at 12 weeks. Restaging PET/CT 2 at 20 weeks demonstrates new areas of FDG uptake in the left side of T9 vertebral body (arrows) and increasing uptake in the left acetabulum, suggesting increasing extent of marrow disease, whereas this is barely seen on CT. Marked physiologic uptake is also seen in brown fat (asterisks).
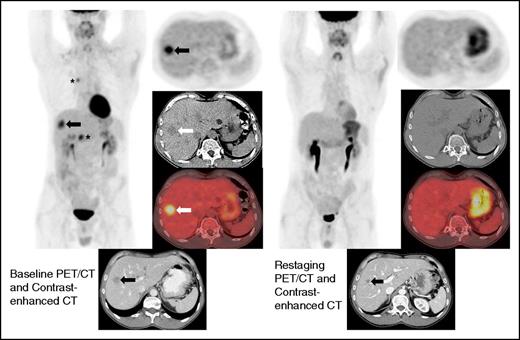
Restaging FDG-PET/CT and contrast-enhanced CT at 19 weeks demonstrates interval resolution of FDG uptake in a liver lesion. Restaging contrast-enhanced CT shows interval decrease in size of the hepatic lesion (arrow). Because the lesion did not disappear, this patient achieved a PR by immune-related response criteria, whereas the absence of FDG uptake on FDG-PET/CT is a CR by the Lugano Classification. There was also a complete metabolic response in the mediastinum and right upper abdomen (asterisks).
Restaging FDG-PET/CT and contrast-enhanced CT at 19 weeks demonstrates interval resolution of FDG uptake in a liver lesion. Restaging contrast-enhanced CT shows interval decrease in size of the hepatic lesion (arrow). Because the lesion did not disappear, this patient achieved a PR by immune-related response criteria, whereas the absence of FDG uptake on FDG-PET/CT is a CR by the Lugano Classification. There was also a complete metabolic response in the mediastinum and right upper abdomen (asterisks).
For all the reasons outlined above, a modification of the Lugano Classification,3,36 notably with regard to the definition of PD, is needed to facilitate the development and maximize the potential therapeutic benefit of new drugs with immunologic mechanisms of action. The Lymphoma Research Foundation, in partnership with the Cancer Research Institute, convened a workshop focusing on the development of response guidelines for lymphomas in the setting of immunomodulatory agents, particularly checkpoint inhibitors. The objectives of the meeting were to address the unique response patterns characteristic of this class of agents and to recommend appropriate adaptations of current lymphoma response criteria. Participants included investigators with experience in the clinical use of checkpoint inhibitors, as well as representatives from companies involved in the development of those agents and from the US Food and Drug Administration. Based on this discussion, we propose a provisional modification of the Lugano criteria adapted to immune-based therapy, the lymphoma response to immunomodulatory therapy criteria (LYRIC). This modification retains the core concepts of IRC summarized above, incorporating them into lymphoma-specific response criteria. This is primarily accomplished through the introduction of a new response category termed indeterminate response (IR). We stress the following points at the outset, which are further discussed in subsequent sections:
This modification is based mostly on the experience with checkpoint blockade therapy, but the framework could potentially be used for other immunomodulatory agents if they are associated with similar atypical response patterns.
The modification is provisional, because we anticipate that future analyses and developments may change or altogether eliminate the IR category. There are not at present sufficient data to rigorously support the details of the choices made here, but such a framework is required to reduce ambiguity in current trials and to enable the collection of accurate data in a consistent way that, eventually, can then be used to support or modify those choices and the incorporation of LYRIC into clinical trials.
IR category
The term IR does not make direct reference to the underlying mechanism, recognizing that a delayed response and an immune-mediated flare can both occur in the early treatment period and may be difficult to distinguish from progression by physical examination or imaging alone. Moreover, the term provides the flexibility to allow patients to continue treatment past IR in some circumstances with a mandatory subsequent evaluation within 12 weeks to confirm or refute true PD.
The following summarizes the provisional definition, nomenclature, suggested management, follow-up, and use of the IR category.
Definition
A patient will be considered to have IR in 1 or more of the 3 following circumstances.
1. Increase in overall tumor burden (as assessed by sum of the product of the diameters [SPD]) of ≥50% of up to 6 measurable lesions in the first 12 weeks of therapy, without clinical deterioration [IR(1)] (Figure 3).
This pattern may be seen as a consequence of either delayed response or early immune-mediated flare. At least within the context of clinical trials, a biopsy is encouraged in this case because this may help to distinguish the two and, if positive, will confirm the impression of PD. However, if negative for lymphoma, it will support the concept of pseudo-progression and contribute to our understanding of this phenomenon. When such a biopsy is neither safe nor feasible, decisions must be based on a repeat scan 12 weeks after the initial determination of IR.
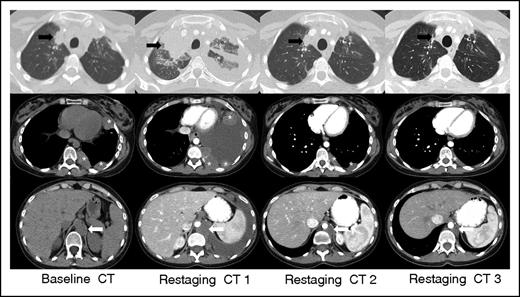
IR(1): Restaging CT 1 at 3 weeks demonstrates overall progression of tumor burden (SPD +124% from baseline) as evidenced interval increase in a right upper lobe lung mass (black arrow), left-sided pleural masses (asterisks), and left retrocrural lymphadenopathy (white arrow), and interval development of a large left-sided pleural effusion. Subsequent follow-up at 7 weeks (restaging CT 2) shows an interval decrease in size of all lesions with resolution of the left pleural effusion (SPD −27% from baseline). Additional follow-up at 13 weeks (restaging CT 3) demonstrates a further interval decrease in tumor burden, and the patient achieved a PR by revised response criteria (SPD −54% from baseline) with clear subsequent clinical benefit from continued treatment.
IR(1): Restaging CT 1 at 3 weeks demonstrates overall progression of tumor burden (SPD +124% from baseline) as evidenced interval increase in a right upper lobe lung mass (black arrow), left-sided pleural masses (asterisks), and left retrocrural lymphadenopathy (white arrow), and interval development of a large left-sided pleural effusion. Subsequent follow-up at 7 weeks (restaging CT 2) shows an interval decrease in size of all lesions with resolution of the left pleural effusion (SPD −27% from baseline). Additional follow-up at 13 weeks (restaging CT 3) demonstrates a further interval decrease in tumor burden, and the patient achieved a PR by revised response criteria (SPD −54% from baseline) with clear subsequent clinical benefit from continued treatment.
It is recognized that “clinical deterioration” is subjective. In some cases, the simple growth of a nodal or tumor mass could worsen the symptoms mechanically related to that mass, such as pain at the tumor site, compression of adjacent structures, etc. Such an increase in symptoms that can be directly attributed to the size of the tumor mass may not be considered as clinical deterioration in this context. However, in most cases, patients should be experiencing clinical stability or improvement by investigator assessment to be considered as having IR, and in all cases, the patient must be considered likely to tolerate continued treatment and not at risk of serious complications should further tumor growth occur.
2. Appearance of new lesions or growth of one or more existing lesion(s) ≥50% at any time during treatment; occurring in the context of lack of overall progression (<50% increase) of overall tumor burden, as measured by SPD of up to 6 lesions at any time during the treatment [IR(2)] (Figure 4).
This phenomenon may occur early or late in the treatment course, and therefore, unlike IR(1), is not defined by its temporal relationship to treatment initiation. Both within and outside the context of clinical trials, a biopsy is strongly encouraged in such cases. If the biopsy does not confirm the presence of viable tumor in the new or enlarging lesion(s), then the lesion(s) are not considered active disease and should not be used in subsequent SPD assessments.
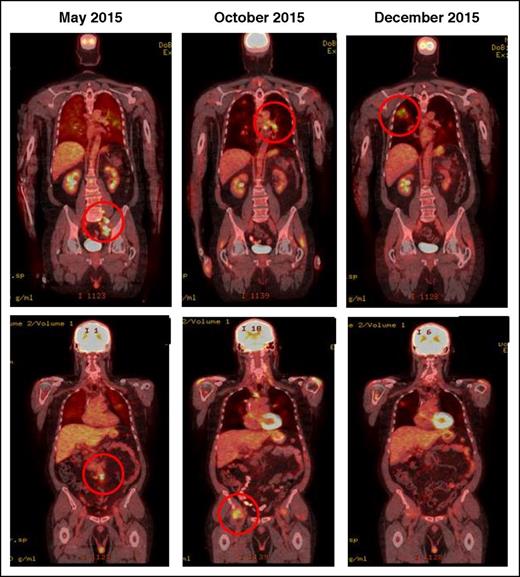
IR(2): CT demonstrating pseudo-progression in a patient on nivolumab for Hodgkin lymphoma. May 2015, pretreatment, October and December 2015 shows transient flares in different nodal groups without overall progression in the original target lesions.
IR(2): CT demonstrating pseudo-progression in a patient on nivolumab for Hodgkin lymphoma. May 2015, pretreatment, October and December 2015 shows transient flares in different nodal groups without overall progression in the original target lesions.
3. Increase in FDG uptake of 1 or more lesion(s) without a concomitant increase in lesion size or number [IR(3)] (Figure 5)
Increased immune activity at the site of tumor may manifest as an increase in FDG uptake. Therefore, by itself, changes in uptake should not trigger an assignment of PD with checkpoint inhibitors. The magnitude of increase in uptake in an immune-mediated flare compared with that in true tumor progression is not yet known. It is important to investigate this finding, especially in conjunction with biopsies of the lesion in question.
IR(3) showing an increase in FDG uptake in a paracardiac node suggestive of lymphoma without a concomitant increase in size of lesion(s) that meets PD criteria.
IR(3) showing an increase in FDG uptake in a paracardiac node suggestive of lymphoma without a concomitant increase in size of lesion(s) that meets PD criteria.
While awaiting a better characterization of this phenomenon, we propose that, under the modified response criteria, an increase in FDG avidity of 1 or more lesions suggestive of lymphoma, without a concomitant increase in size of those lesions meeting PD criteria does not constitute PD.
It is possible that, at a single time point, a patient could fulfill criteria for both [IR(1)] or [IR(2)] and [IR(3)]: for example, there could be a new FDG avid lesion in the absence of overall progression [IR(2)], and, at the same time, increase in FDG uptake of a separate lesion [IR(3)]. In such cases, the designation of [IR(1 or 2)] should take priority [eg, IR(2)] in the above example].
These 3 patterns of IR as defined above [ie, IR(1), IR(2), and IR(3)] may have very different mechanisms and clinical implications. Therefore, it is critical that data are collected in a consistent manner so that these 3 possible atypical response types occurring within the context of checkpoint inhibitors can be distinguished.
Follow-up of IR
In patients categorized as having any of the above types of IR, it is mandatory to obtain a repeat imaging after an additional 12 weeks (or earlier if clinically indicated). At that time, response should be re-evaluated, and the patient should be considered to have true PD if the SPD of target lesion has increased further, with the considerations below:
In the case of IR(1), the comparison should be between the first IR(1) and the current SPD, with an increase of ≥10% constituting PD. In addition, there should be an increase of ≥5 mm (in either dimension) of ≥1 lesion for lesions ≤2 cm and 10 mm for lesions >2 cm, to be consistent with the Lugano classification3 (Table 2). The 10% threshold is empiric but designed to account for variability in measurement,37 especially when taken along with the minimum increase. If the target SPD increase is <10%, the response would still be categorized as IR(1), and the patient could continue treatment until a subsequent scan shows either true PD [≥10% increase from first IR(1) time point and an increase of >5 mm in either dimension of ≥1 lesion] or response (≥50% decrease from baseline). In this situation, it is reasonable to repeat imaging in 4 to 8 weeks of the original IR(1) time point to ensure absence of significant further increase.
In the case of IR(2), the new or growing lesion(s) (unless biopsy proven to be benign) should be added to the target lesion(s), up to a total of no more than 6 total lesions. If the SPD of the newly defined set of target lesions has increased ≥50% from their nadir value (which may precede the IR time point), the patient should be considered to have PD.
In the case of IR(3), because inflammatory responses may result in an increase in the standardized uptake value of a lesion, the patient will not be considered to have PD unless there is evidence of PD by an increase in lesion size or the development of new lesions, as noted above.
Comparison of RECIST, irRC, and Lugano Classification criteria
| Criteria . | CR . | PR . | PD . |
|---|---|---|---|
| RECIST 1.1 | Disappearance of all target lesions. Any pathological lymph nodes (whether target or nontarget) must have reduction in short axis to <10 mm | At least a 30% decrease in the sum of diameters of target lesions, taking as reference the baseline sum diameters | At least a 20% increase in the sum of diameters of target lesions, taking as reference the smallest sum on study (this includes the baseline sum if that is the smallest on study). In addition to the relative increase of 20%, the sum must also demonstrate an absolute increase of at least 5 mm |
| Note: the appearance of one or more new lesions is also considered progression. | |||
| irRC | Disappearance of all lesions in two consecutive observations not less than 4 weeks apart | ≥50% decrease in tumor burden compared with baseline in 2 observations at least 4 weeks apart (as measured bidimensionally) | ≥25% increase in tumor burden compared with nadir (at any single time point) in 2 consecutive observations at least 4 weeks apart, where Tumor Burden = SPD index lesions + SPD new, measurable lesions |
| Lugano | PET-CT, score 1, 2, or 3* with or without a residual mass on 5PS† OR on CT, target nodes/nodal masses must regress to ≤1.5 cm in LDi | PET-CT score 4 or 5 with reduced uptake compared with baseline and residual mass(es) of any size. OR On CT ≥50% decrease in SPD of up to 6 target measurable nodes and extranodal sites | PET-CT score 4 or 5 with an increase in intensity of uptake from baseline and/or new FDG-avid foci consistent with lymphoma at interim or end-of-treatment assessment. OR On CT, an individual node/lesion must be abnormal with: LDi >1.5 cm and increase by ≥50% from PPD nadir and an increase in LDi or SDi from nadir 0.5 cm for lesions ≤2 cm 1.0 cm for lesions >2 cm |
| In the setting of splenomegaly, the splenic length must increase by >50% of the extent of its prior increase beyond baseline (eg, a 15-cm spleen must increase to >16 cm). If no prior splenomegaly, must increase by ≥2 cm from baseline. New or recurrent splenomegaly | |||
| New or clear progression of preexisiting nonmeasured lesions | |||
| Regrowth of previously resolved lesions | |||
| A new node >1.5 cm in any axis or a new extranodal site >1.0 cm in any axis; if <1.0 cm in any axis, its presence must be unequivocal and must be attributable to lymphoma | |||
| Assessable disease of any size unequivocally attributable to lymphoma | |||
| AND/OR new or recurrent involvement of the bone marrow | |||
| LYRIC | Same as Lugano | Same as Lugano | As with Lugano with the following exceptions: |
| IR | |||
| IR(1): ≥50% increase in SPD in first 12 weeks | |||
| IR(2): <50% increase in SPD with | |||
| a. New lesion(s), or | |||
| b. ≥50% increase in PPD of a lesion or set of lesions at any time during treatment | |||
| IR(3): Increase in FDG uptake without a concomitant increase in lesion size meeting criteria for PD |
| Criteria . | CR . | PR . | PD . |
|---|---|---|---|
| RECIST 1.1 | Disappearance of all target lesions. Any pathological lymph nodes (whether target or nontarget) must have reduction in short axis to <10 mm | At least a 30% decrease in the sum of diameters of target lesions, taking as reference the baseline sum diameters | At least a 20% increase in the sum of diameters of target lesions, taking as reference the smallest sum on study (this includes the baseline sum if that is the smallest on study). In addition to the relative increase of 20%, the sum must also demonstrate an absolute increase of at least 5 mm |
| Note: the appearance of one or more new lesions is also considered progression. | |||
| irRC | Disappearance of all lesions in two consecutive observations not less than 4 weeks apart | ≥50% decrease in tumor burden compared with baseline in 2 observations at least 4 weeks apart (as measured bidimensionally) | ≥25% increase in tumor burden compared with nadir (at any single time point) in 2 consecutive observations at least 4 weeks apart, where Tumor Burden = SPD index lesions + SPD new, measurable lesions |
| Lugano | PET-CT, score 1, 2, or 3* with or without a residual mass on 5PS† OR on CT, target nodes/nodal masses must regress to ≤1.5 cm in LDi | PET-CT score 4 or 5 with reduced uptake compared with baseline and residual mass(es) of any size. OR On CT ≥50% decrease in SPD of up to 6 target measurable nodes and extranodal sites | PET-CT score 4 or 5 with an increase in intensity of uptake from baseline and/or new FDG-avid foci consistent with lymphoma at interim or end-of-treatment assessment. OR On CT, an individual node/lesion must be abnormal with: LDi >1.5 cm and increase by ≥50% from PPD nadir and an increase in LDi or SDi from nadir 0.5 cm for lesions ≤2 cm 1.0 cm for lesions >2 cm |
| In the setting of splenomegaly, the splenic length must increase by >50% of the extent of its prior increase beyond baseline (eg, a 15-cm spleen must increase to >16 cm). If no prior splenomegaly, must increase by ≥2 cm from baseline. New or recurrent splenomegaly | |||
| New or clear progression of preexisiting nonmeasured lesions | |||
| Regrowth of previously resolved lesions | |||
| A new node >1.5 cm in any axis or a new extranodal site >1.0 cm in any axis; if <1.0 cm in any axis, its presence must be unequivocal and must be attributable to lymphoma | |||
| Assessable disease of any size unequivocally attributable to lymphoma | |||
| AND/OR new or recurrent involvement of the bone marrow | |||
| LYRIC | Same as Lugano | Same as Lugano | As with Lugano with the following exceptions: |
| IR | |||
| IR(1): ≥50% increase in SPD in first 12 weeks | |||
| IR(2): <50% increase in SPD with | |||
| a. New lesion(s), or | |||
| b. ≥50% increase in PPD of a lesion or set of lesions at any time during treatment | |||
| IR(3): Increase in FDG uptake without a concomitant increase in lesion size meeting criteria for PD |
IR, immune response; LDi, longest diameter; PPD, product of the perpendicular diameters; SDi, short diameter; 5PS, 5-point scale.
A score of 3 in many patients indicates a good prognosis with standard treatment, especially if at the time of an interim scan. However, in trials involving PET where de-escalation is investigated, it may be preferable to consider a score of 3 as inadequate response (to avoid undertreatment).
PET 5PS: 1, no uptake above background; 2, uptake ≤ mediastinum; 3, uptake > mediastinum but ≤ liver; 4, uptake greater than liver; 5, uptake markedly higher than liver (2-3 times SUVmax in normal liver) and/or new lesions; X, new areas of uptake unlikely to be related to lymphoma.
Importantly, if a patient is assessed as having IR and then “true” PD at a subsequent time point (without an intervening objective response between IR and PD), the IR assessment should subsequently be corrected to PD for reporting purposes to the date of the prior designation of IR. We recognize that these lesions may remain stable during the time of observation, but, even if this is the case, the initial designation of IR should be changed to PD.
Use of the IR category
We propose that the modified response criteria outlined here be incorporated as secondary end points in upcoming clinical trials of immunomodulatory therapy (especially trials involving checkpoint blockade). Moreover, we propose that the protocols using such agents allow the use of treatment past conventional PD, in the case of IR, as outlined in above. This will not only allow patients to continue what may be beneficial therapy, but will allow the generation of data that can then be analyzed to determine whether this strategy of treatment past IR does indeed confer a clinical benefit, in a way similar to what has been done in solid tumors.
Conclusions
Despite the recently revised response criteria for lymphoma,3 modifications in treatment that affect image interpretation demand continued revision of the response criteria to optimize drug development and patient management. Moreover, the question of how best to apply response criteria in an era increasingly focused on targeted treatments with prolonged duration of therapy has yet to be resolved, as does the question of when best to assess response in an era of continuous treatments.
Immune-modulating active agents are now entering the clinic in lymphoma in increasing numbers and are likely to provide a valuable addition to our therapeutic arsenal. Given the growing utilization of these agents, optimizing response assessment will be critical to maximize potential therapeutic benefit. We hope that the addition of the IR category to standard response assessment will allow investigators to better understand this phenomenon, gain insights to the biologic bases of response and flare, allow patients to derive maximal possible benefit from these drugs, and further refine immune-related response criteria for their application in hematologic malignancies as our knowledge matures. Once we enlarge our data set and understanding, and can accurately distinguish delayed responses and flares from PD, the provisional term IR should disappear as has the former complete remission unconfirmed, to not artificially influence response rates.
Although the foregoing focused primarily on checkpoint blocking agents, similar considerations could potentially apply to other immunotherapies such as bi-specific antibodies, engineered T cells, and others. In the future, novel imaging methods and quantitative methods to measure tumor burden may also prove helpful in response assessment. There is currently growing interest in the use of next-generation sequencing techniques for detecting minimal residual disease in a number of lymphoma subtypes and studies are evaluating the role of circulating tumor cell DNA for response assessment as well.38,39
Ultimately, it will always be challenging to match our assessment tools to our treatments. This ongoing effort is critical to maximize the early detection of treatment failure while limiting the possibility of discarding a useful treatment too soon. Only through the continuous pursuit of this goal can we succeed in optimizing patient outcome.
Acknowledgments
The authors thank Richard I. Fisher. T. Andrew Lister, and Emanuele Zucca for critical review of the manuscript and Fernanda C. Cabral and Annick D. Van Den Abbeele for provision of images. Most notably, we appreciate the Lymphoma Research Foundation and the Cancer Research Institute for their sponsoring of the workshop leading to these recommendations.
Authorship
Contribution: B.D.C., S.A., L.S., L.I.G., R.A., A.H., and P.A. participated in the workshop. All of the authors were involved in writing the manuscript and its final approval.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Bruce D. Cheson, Georgetown University Hospital, Lombardi Comprehensive Cancer Center, Washington, DC 20007; e-mail: bdc4@georgetown.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal