Key Points
Children with sickle cell disease engrafted unrelated donor marrow after reduced intensity conditioning.
A high incidence of GVHD and associated mortality compromised safety of the trial.
Abstract
Children with sickle cell disease experience organ damage, impaired quality of life, and premature mortality. Allogeneic bone marrow transplant from an HLA-matched sibling can halt disease progression but is limited by donor availability. A Blood and Marrow Transplant Clinical Trials Network (BMT CTN) phase 2 trial conducted from 2008 to 2014 enrolled 30 children aged 4 to 19 years; 29 were eligible for evaluation. The primary objective was 1-year event-free survival (EFS) after HLA allele-matched (at HLA-A, -B, -C, and -DRB1 loci) unrelated donor transplant. The conditioning regimen included alemtuzumab, fludarabine, and melphalan. Graft-versus-host disease (GVHD) prophylaxis included calcineurin inhibitor, short-course methotrexate, and methylprednisolone. Transplant indications included stroke (n = 12), transcranial Doppler velocity >200 cm/s (n = 2), ≥3 vaso-occlusive pain crises per year (n = 12), or ≥2 acute chest syndrome episodes (n = 4) in the 2 years preceding enrollment. Median follow-up was 26 months (range, 12-62 months); graft rejection was 10%. The 1- and 2-year EFS rates were 76% and 69%, respectively. The corresponding rates for overall survival were 86% and 79%. The day 100 incidence rate of grade II-IV acute GVHD was 28%, and the 1-year incidence rate of chronic GVHD was 62%; 38% classified as extensive. There were 7 GVHD-related deaths. A 34% incidence of posterior reversible encephalopathy syndrome was noted in the first 6 months. Although the 1-year EFS met the prespecified target of ≥75%, this regimen cannot be considered sufficiently safe for widespread adoption without modifications to achieve more effective GVHD prophylaxis. The BMT CTN #0601 trial was registered at www.clinicaltrials.gov as #NCT00745420.
Introduction
Sickle cell disease (SCD) is a monogenic hemoglobin disorder characterized by hemolytic anemia with variable clinical manifestations after endothelial damage and vasculopathy.1 Hypoperfusion results in multiple organ damage. In patients with severe disease, symptoms manifest early and progress during childhood. Allogeneic hematopoietic cell transplantation can replace sickle erythropoiesis. The results of HLA-matched sibling donor transplants are excellent, with event-free survival (EFS) in excess of 90% and with acceptable rates of graft rejection (GR) and graft-versus-host disease (GVHD).2-5 HLA-matched sibling donor transplants account for the majority of transplants performed worldwide for hemoglobinopathy.6,7 However, only 18% of patients with SCD have an HLA-matched sibling donor in the United States.8 HLA-matched adult unrelated donors (URDs) have been used to expand the donor pool for nonmalignant hematologic disorders, but their role in SCD transplants is unclear.9-11 Although the likelihood of finding an HLA-matched URD for African Americans is low at 16% to 19%, utilization of these donors does expand the donor pool.12 To date, most SCD transplants have used myeloablative conditioning regimens, but these can result in toxicities such as growth inhibition, gonadal hypofunction, and sterility.13-16 Reduced-intensity conditioning (RIC) regimens, although associated with a more favorable toxicity profile, can be associated with higher rates of GR, especially with graft sources such as umbilical cord blood.17-20 A RIC regimen augmented with host immunoablation by alemtuzumab was previously successful in achieving donor engraftment.21,22 In that report of HLA-matched sibling donor bone marrow transplant (BMT) in 52 children with hemoglobinopathies, acute and chronic GVHD rates were 23% and 13%, respectively.21 The regimen was adopted for a phase 2 URD transplant trial with bone marrow or umbilical cord blood grafts through the Blood and Marrow Transplant Clinical Trials Network (BMT CTN #0601; NCT 00745420). This report describes outcomes from the trial using bone marrow grafts. The umbilical cord blood arm was closed early after a high GR rate.19
Methods
Study design
The primary end point was 1-year EFS; death from any cause, GR, or recurrent disease was considered an event. Prespecified secondary end points included overall survival, hematopoietic recovery, acute and chronic GVHD, infections, hepatic sinusoidal syndrome, interstitial pneumonitis, seizure, posterior reversible encephalopathy syndrome (PRES), and health-related quality of life (HRQL). The trial opened on 11 April 2008 and closed to enrollment on 24 April 2014. Enrollment was paused once for clarification of HLA typing requirements once (for 6 months) during this period. This analysis includes data collected as of March 2016. The median follow-up of surviving patients was 26 months (range, 12-62 months). All patients were followed for at least 24 months, except for 1 patient who was lost to follow-up at 12 months.
Patients
The protocol was approved by the institutional review board at each of the participating institutions. Informed consent was obtained from parents or from patients aged >18 years, and assent was obtained from patients aged 7-18 years before enrollment. The consent form extensively described alternate conservative treatment approaches for SCD, as well as the pros and cons of transplant. Trial eligibility was confirmed by 3 independent hematologists. Eligible patients were aged 3.0 to 20 years who had severe SCD indicated by 1 or more the following: (1) clinically significant neurologic event (stroke) or any neurologic defect lasting >24 hours and accompanied by an infarct on cerebral magnetic resonance imaging; (2) a transcranial Doppler velocity >200 cm/s by the nonimaging technique, or velocity that exceeded 185 cm/s by the imaging technique measured at a minimum of 2 separate occasions 1 month or more apart23 ; (3) a minimum of 2 episodes of acute chest syndrome within the preceding 2-year period and defined as new pulmonary alveolar consolidation involving at least 1 complete lung segment despite adequate supportive care measures; and (4) minimum of 3 new pain events per year in the previous 2 years and defined as new onset of pain lasting for at least 2 hours, for which there was no other explanation, and occurred despite adequate supportive care measures. Adequate organ function pretransplant required the following: serum creatinine levels <1.5 times the upper limit of normal for age and the glomerular filtration rate >100 mL/min per 1.73 m2 or adjusted for age; alanine aminotransferase and aspartate aminotransferase <5 times the upper limit of normal and direct serum bilirubin <2 times the upper limit of normal; left ventricular ejection fraction >40% or left ventricular shortening fraction >26%; and diffusing capacity of the lungs for carbon monoxide >40% of predicted (corrected for hemoglobin). Patients with serum ferritin >1000 ng/mL were required to have a liver biopsy demonstrating the absence of cirrhosis and bridging fibrosis if they had received regular red cell transfusions for >1 year. Hemoglobin S level was maintained at ≤45% within 7 days of initiation of transplant conditioning, and chelation and/or hydroxyurea were discontinued 48 hours before initiation of conditioning. Ineligible patients included those with an HLA-matched sibling, HIV seropositivity, a performance score <40, and uncontrolled bacterial, viral, or fungal infection.
Treatment
The conditioning regimen included alemtuzumab, fludarabine, and melphalan, with the alemtuzumab administered between day 22 and day 18 before graft infusion to achieve host immunoablation (Table 1). Prophylaxis for GVHD consisted of a calcineurin inhibitor (tacrolimus or cyclosporine) administered from day −3 through day 100 after graft infusion, with subsequent taper through day 180; methotrexate 7.5 mg/m2 on days 1, 3, and 6; and methylprednisolone 1 mg/kg per day from days 7 through 28, with subsequent taper by 20% per week. Supportive care recommendations included granulocyte colony-stimulating factor commenced on day 7 and continued until an absolute neutrophil count of 1.5 × 109/L on 3 days after the nadir, weekly surveillance for cytomegalovirus and Epstein-Barr virus reactivation, seizure prophylaxis for the duration of use of calcineurin inhibitors, strict blood pressure control, preemptive therapy for viral infections, bacterial prophylaxis through day 100, and prompt treatment of overt or suspected infections. To mitigate the risk of intracranial hemorrhage, platelet count was maintained at ≥50 × 109/L.24
Treatment regimen
| Day . | Treatment . |
|---|---|
| −23 | Alemtuzumab test dose 3 mg IV* |
| −22 | Alemtuzumab 10 mg IV† |
| −21 | Alemtuzumab 15 mg IV† |
| −20 | Alemtuzumab 20 mg IV† |
| −8 | Fludarabine 30 mg/m2 IV |
| −7 | Fludarabine 30 mg/m2 IV |
| −6 | Fludarabine 30 mg/m2 IV |
| −5 | Fludarabine 30 mg/m2 IV |
| −4 | Fludarabine 30 mg/m2 IV |
| −3 | Melphalan 140 mg/m2 IV; cyclosporine or tacrolimus dosed to maintain appropriate levels through day 100, and then tapered to day 180 |
| −2 | Rest |
| −1 | Rest |
| 0 | Bone marrow infusion |
| 1 | Methotrexate 7.5 mg/m2 IV |
| 3 | Methotrexate 7.5 mg/m2 IV |
| 6 | Methotrexate 7.5 mg/m2 IV |
| 7 | Methylprednisolone 1 mg/kg per day IV through day 28, and then taper granulocyte colony-stimulating factor 5 μg/kg body weight per day IV until absolute neutrophil count is ≥0.5 × 109/L for 3 consecutive days |
| Day . | Treatment . |
|---|---|
| −23 | Alemtuzumab test dose 3 mg IV* |
| −22 | Alemtuzumab 10 mg IV† |
| −21 | Alemtuzumab 15 mg IV† |
| −20 | Alemtuzumab 20 mg IV† |
| −8 | Fludarabine 30 mg/m2 IV |
| −7 | Fludarabine 30 mg/m2 IV |
| −6 | Fludarabine 30 mg/m2 IV |
| −5 | Fludarabine 30 mg/m2 IV |
| −4 | Fludarabine 30 mg/m2 IV |
| −3 | Melphalan 140 mg/m2 IV; cyclosporine or tacrolimus dosed to maintain appropriate levels through day 100, and then tapered to day 180 |
| −2 | Rest |
| −1 | Rest |
| 0 | Bone marrow infusion |
| 1 | Methotrexate 7.5 mg/m2 IV |
| 3 | Methotrexate 7.5 mg/m2 IV |
| 6 | Methotrexate 7.5 mg/m2 IV |
| 7 | Methylprednisolone 1 mg/kg per day IV through day 28, and then taper granulocyte colony-stimulating factor 5 μg/kg body weight per day IV until absolute neutrophil count is ≥0.5 × 109/L for 3 consecutive days |
The test dose of alemtuzumab was administered 24 hours before the first dose, shown in this example on day −23.
Alemtuzumab treatment was required to be administered on 3 consecutive days but could begin on day −22, −21, or −20 and end on day −20, −19, or −18, respectively.
Outcomes
The primary end point was 1-year EFS. Primary or secondary GR or death was considered an event. Primary GR was defined as the presence of <20% donor cells as assessed by bone marrow or peripheral blood chimerism assays (any lineage) on or after day 42. Secondary GR was defined as the presence of <20% donor-derived hematopoietic cells in peripheral blood or bone marrow in a patient with prior evidence of >20% donor cells. The level was chosen based on absence of SCD symptoms, even when donor chimerism in blood or marrow approached 10%.21,25 Survival was defined as the time from transplant to death or last follow-up. Neutrophil recovery was defined as the first of 3 days when the absolute neutrophil count was ≥0.5 × 109/L. Platelet recovery was defined as the first of 7 days without a platelet transfusion that the platelet count was ≥50 × 109/L. Acute and chronic GVHD were graded by Seattle (ie, limited/extensive) and National Institutes of Health criteria (ie, mild/moderate/severe).26 Common Terminology Criteria for Adverse Events version 3.0 was used to report expected grade 3-5 adverse events. Major regimen-related toxicity (RRT) was defined as grade 4 or 5 in any organ system or grade 3 for pulmonary, cardiac, renal, central nervous system, oral, or mucosal.27 HRQL was assessed pretransplant (within 2 months of transplant), and thereafter on day 100, at 6 months, and at 1 year posttransplant using the Child Health Questionnaire (CHQ). The CHQ was chosen because it was the only HRQL measure that was validated and reliable for use in children with SCD at the time the study commenced.28,29 The CHQ-Parent Form 50 was used for parent reports, and the CHQ-Child Form 87 was used for the child self-report for children aged ≥10 years.
Statistical analysis
The primary hypothesis was that a RIC regimen would be sufficient for stable engraftment after HLA-matched URD BMT and result in 1-year EFS ≥75%. The sample size of 30 patients was chosen based on a 95% confidence interval (CI) length of 31. However, 1 patient was deemed ineligible after enrollment because the patient and donor were mismatched at 2 HLA-loci. Therefore the analysis includes 29 patients.
EFS and overall survival were calculated using the Kaplan-Meier estimator.30 The cumulative incidence method was used to estimate the incidence of events in the presence of competing risks for neutrophil and platelet recovery and acute and chronic GVHD; in each case, death was considered the competing risk.31 HRQL measurement was based on the CHQ Parent Form 50 (for patients 5-18 years old) and the CHQ Child Form 87 (for patients 10-18 years old). Mean scores for the CHQ were calculated based on a 4- to 6-point response scale for each item and transformed according to the developer’s instructions to a 0 to 100 scale, with a higher score representing a better quality of life.32 One domain, Change in Health, is composed of 1 question that is reported on a 1- to 5-point scale, with a higher number meaning better health. The impact of a change by >1 point is considered significant on a 5-category scale.33,34 HRQL data were analyzed for changes in mean HRQL score from pretransplant measurement and performed as an exploratory analysis, given the small sample size. A paired Student t test was used to assess changes from baseline to each posttransplant time point (day 100, 6 months, and 1 year). Only P values of <.01 were considered significant for the HRQL analyses, given the multiple comparisons. All analyses were performed using SAS version 9.3 (Cary, NC).
Results
Patient and donor characteristics
The characteristics of the donors and 29 patients who met eligibility criteria are shown in Table 2. All patients had a hemoglobin SS genotype. The median age at transplant was 14 years (range, 6-19 range), and 55% were male. Indications for transplant included stroke (41%), elevated transcranial Doppler velocity (7%), recurrent episodes of acute chest syndrome (14%), or significant pain (41%). Pretransplant, 26 patients had performance scores of 90 or 100, 2 patients had a score of 80, and the remaining patient had a score of 70. All patients had received erythrocyte transfusions before transplant. The median serum ferritin level was 722 ng/mL (range, 55-7324 ng/mL). Eight patients underwent liver biopsy for history of chronic red cell transfusions and a serum ferritin level of >1000 ng/mL, but no patient was excluded because none had evidence of bridging fibrosis or liver cirrhosis. The median hemoglobin S at transplant was 20.8% (range, 3.9%-43%). All 29 eligible patients received bone marrow grafts from an adult URD and were HLA matched at the allele-level at HLA-A, -B, -C, and -DRB1. One unrelated adult donor was a carrier with hemoglobin AS genotype. The median total nucleated cell dose of the bone marrow graft was 3.5 × 108/kg (range, 1.3 × 108/kg to 6.8 × 108/kg). The median CD34 dose of the graft was 2.9 × 106/kg (range, 0.3 × 106/kg to 9.2 × 106/kg).
Donor, recipient, and transplant characteristics
| Characteristics . | n . |
|---|---|
| Donor* | |
| Race/ethnicity | |
| White | 11 |
| African American | 11 |
| Multiracial | 5 |
| Not reported | 2 |
| Recipient† | |
| Sex | |
| Male | 16 |
| Female | 13 |
| Race/ethnicity | |
| African American | 26 |
| Hispanic | 3 |
| Indications for transplant‡ | |
| Stroke | 12 |
| Transcranial Doppler velocity >200 cm/s | 2 |
| Acute chest syndrome | 4 |
| Vaso-occlusive pain crisis | 12 |
| Chronic blood transfusion before transplant | 14 |
| Performance score | |
| 100 | 17 |
| 90 | 9 |
| 80 | 2 |
| 70 | 1 |
| Transplant | |
| CMV-seronegative donor and recipient | 8 |
| CMV-seropositive donor and recipient | 9 |
| CMV-seronegative donor and CMV-seropositive recipient | 3 |
| CMV-seropositive donor and CMV-seronegative recipient | 9 |
| ABO blood group matched | 15 |
| ABO blood group major mismatch | 9 |
| ABO blood group minor mismatch | 5 |
| Sex-matched transplants | 15 |
| Female donor, male recipient | 7 |
| Male donor, female recipient | 7 |
| Characteristics . | n . |
|---|---|
| Donor* | |
| Race/ethnicity | |
| White | 11 |
| African American | 11 |
| Multiracial | 5 |
| Not reported | 2 |
| Recipient† | |
| Sex | |
| Male | 16 |
| Female | 13 |
| Race/ethnicity | |
| African American | 26 |
| Hispanic | 3 |
| Indications for transplant‡ | |
| Stroke | 12 |
| Transcranial Doppler velocity >200 cm/s | 2 |
| Acute chest syndrome | 4 |
| Vaso-occlusive pain crisis | 12 |
| Chronic blood transfusion before transplant | 14 |
| Performance score | |
| 100 | 17 |
| 90 | 9 |
| 80 | 2 |
| 70 | 1 |
| Transplant | |
| CMV-seronegative donor and recipient | 8 |
| CMV-seropositive donor and recipient | 9 |
| CMV-seronegative donor and CMV-seropositive recipient | 3 |
| CMV-seropositive donor and CMV-seronegative recipient | 9 |
| ABO blood group matched | 15 |
| ABO blood group major mismatch | 9 |
| ABO blood group minor mismatch | 5 |
| Sex-matched transplants | 15 |
| Female donor, male recipient | 7 |
| Male donor, female recipient | 7 |
CMV, cytomegalovirus.
Median donor age was 35 years (range, 21-55 years).
Median recipient age was 14 years (range, 6-19 years).
Some patients had more than 1 indication.
EFS, overall survival, and engraftment
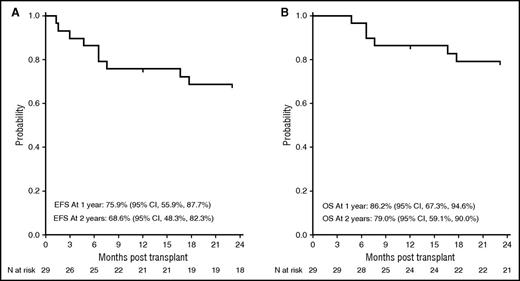
The primary end point was met. The 1-year EFS was 76% (95% CI, 56-88) and the 1-year overall survival was 86% (95% CI, 67-95). At data cutoff in March 2016, the 2-year EFS was 69% (95% CI, 48-82) and the 2-year overall survival was 79% (95% CI, 59-90) (Figure 1). Twenty-seven of 29 patients engrafted. The median time to neutrophil recovery was 12 days (range, 6-16 days) and the median time to platelet recovery was 24 days (range, 7-90 days), similar to published results using URDs.35 Two patients experienced primary GR (day 39 and day 91); 1 patient developed secondary GR on day 48 for a cumulative incidence of GR of 10%. All 3 patients experiencing GR recovered host hematopoiesis without marrow aplasia. Because mixed chimerism occurs frequently after transplant for SCD, we evaluated the percentage of donor cells at 3 months, at 1 year, and at 2 years.20,21,25 All engrafted patients demonstrated >90% donor chimerism at 3 months, and this persisted at the 1-year and 2-year time points in evaluable patients (n = 22 and n = 19 at 1 and 2 years, respectively). Hemoglobin S levels were undetectable in all but 1 patient who received a graft from an URD with sickle cell trait, and the hemoglobin S level was consequently 42%.
Probability of EFS and overall survival. The 2-year probability of EFS (A) and overall survival (B) after URD transplant for severe SCD.
Probability of EFS and overall survival. The 2-year probability of EFS (A) and overall survival (B) after URD transplant for severe SCD.
Although a RIC regimen was administered, RRT was implicated in 83% of grade 3-5 adverse events (63/76). Ten patients developed PRES with a 1-year incidence of 34% (95% CI, 18-52), resulting in reiteration of the importance of strict blood pressure control based on lower blood pressure norms established for SCD patients and of correcting any electrolyte imbalance. Two PRES events occurred before transplant. In the remaining 8 patients, calcineurin inhibitor was withdrawn. Thereafter, 1 patient received sirolimus, 2 received mycophenolate mofetil, and 5 received no alternate GVHD prophylaxis. Three patients developed renal failure and required dialysis. These events were transient, and all patients fully recovered.
Eight patients died after transplant. Seven patients, all aged ≥14 years, died of GVHD and related complications (Table 3). Five of the 7 patients had ferritin levels >1000 ng/mL, although none had fibrosis or cirrhosis. One patient with primary GR died of infection 3 months after a second myeloablative transplant. Four GVHD-related deaths occurred within the first year after transplant, whereas 3 occurred between 507 and 960 days.
Cause of death in 7 patients
| Patient age, y . | Time of death, d . | Complications at the time of death . |
|---|---|---|
| 17 | 231 | Acute GVHD (gut); opportunistic infection; ARDS |
| 16 | 539 | Chronic GVHD; CMV infection; encephalomyelitis; cardiorespiratory failure |
| 17 | 200 | Acute GVHD (gut); respiratory and renal failure |
| 19 | 960 | Chronic GVHD |
| 16 | 507 | Chronic GVHD; VRE and HSV infections |
| 14 | 199 | Acute GVHD; Candida and CMV infections; respiratory and renal failure |
| 18 | 143 | Acute GVHD (gut); pulmonary hemorrhage; Staphylococcus aureus pneumonia |
| Patient age, y . | Time of death, d . | Complications at the time of death . |
|---|---|---|
| 17 | 231 | Acute GVHD (gut); opportunistic infection; ARDS |
| 16 | 539 | Chronic GVHD; CMV infection; encephalomyelitis; cardiorespiratory failure |
| 17 | 200 | Acute GVHD (gut); respiratory and renal failure |
| 19 | 960 | Chronic GVHD |
| 16 | 507 | Chronic GVHD; VRE and HSV infections |
| 14 | 199 | Acute GVHD; Candida and CMV infections; respiratory and renal failure |
| 18 | 143 | Acute GVHD (gut); pulmonary hemorrhage; Staphylococcus aureus pneumonia |
ARDS, acute respiratory distress syndrome; CMV, cytomegalovirus; HSV, herpes simplex virus; VRE, vancomycin-resistant Enterococcus.
GVHD
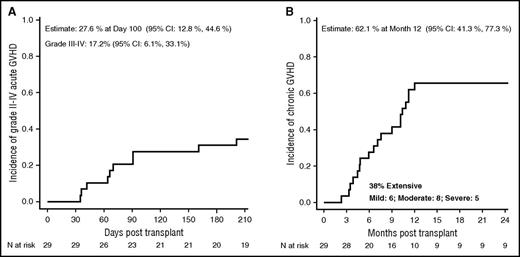
The cumulative incidence on day 100 of grade II-IV acute GVHD was 28% (95% CI, 13-45), and was 17% (95% CI, 6-33) for grade III-IV acute GVHD (Figure 2A). The cumulative incidence of chronic GVHD at 1 year (Figure 2B) was 62% (95% CI, 41-77), with 38% classified as extensive. By National Institutes of Health scoring criteria, chronic GVHD was classified as mild in 6 patients, moderate in 8, and severe in 5. Of the 19 patients with sustained donor engraftment, 4 discontinued immune suppression by 1 year, 6 discontinued in the second year, and 5 discontinued after 2 years. Of the remaining 4 patients, 1 was weaning immune suppression when lost to follow-up at 1 year, 2 were weaning post-GVHD resolution, and 1 continued treatment of stable chronic GVHD. Performance scores at the 2-year or last follow-up visit were 100 in 13 patients, 90 in 5 patients, and 80 in 3 patients.
Probability of GVHD. The 100-day probability of acute GVHD (A) and the 1-year probability of GVHD (B) after URD transplant for severe SCD.
Probability of GVHD. The 100-day probability of acute GVHD (A) and the 1-year probability of GVHD (B) after URD transplant for severe SCD.
HRQL
SCD adversely affects quality of life, as previously reported.36 We were interested in learning whether HRQL improved after URD BMT in pediatric recipients. Validated measures for HRQL include change in health, physical functioning, behavior, and self-esteem. Parental proxies and patients who completed the forms, as indicated based on age, reported significant improvements in the change in health domain posttransplant (Table 4). Although initially, patients did not report any differences, parent proxies (n = 21) reported significantly worse Self-Esteem HRQL scores (mean change, −15.12; P = .006) but significantly better General Health Perception scores (mean change, 11.13; P = .0003) at day 100 compared with pretransplant baseline. The child-reported Change in Health score (n = 13) improved by a mean of 1.46 (P = .0013) at 12 months posttransplant compared with pretransplant scores. Parental proxies reported similar improvements at 6 and 12 months compared with pretransplant scores (mean change, 1.15 and 1.53, respectively; P < .01). The limited sample size precluded subanalyses such as assessing these changes in patients with and without chronic GVHD (see supplemental Table 1, available on the Blood Web site).
HRQL changes from pretransplant baseline to day 100, 6 months, and 1 year
| HRQL score . | Day 100 . | 6 Months . | 1 Year . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| n . | Mean (SEM) . | P . | n . | Mean (SEM) . | P . | n . | Mean (SEM)* . | P . | |
| Parent proxy | |||||||||
| Self-Esteem | 21 | −15.12 (4.89) | .0057* | 20 | −11.46 (6.28) | .0839 | 15 | −3.61 (6.66) | .5959 |
| General Health Perception | 21 | 11.13 (2.51) | .0003* | 20 | 7.63 (3.31) | .0326 | 15 | 8.89 (5.27) | .1135 |
| Change in Health | 21 | 0.90 (0.38) | .0286 | 20 | 1.15 (0.36) | .0052* | 15 | 1.53 (0.48) | .0062* |
| Child | |||||||||
| Self-Esteem | 18 | −0.59 (4.51) | .8976 | 16 | −4.33 (5.87) | .4722 | 13 | 4.97 (5.33) | .3691 |
| General Health Perception | 18 | −1.90 (4.29) | .6631 | 16 | −6.69 (4.99) | .2001 | 13 | 6.99 (6.35) | .2927 |
| Change in Health | 18 | 0.33 (0.45) | .4691 | 16 | 0.19 (0.52) | .7225 | 13 | 1.46 (0.35) | .0013* |
| HRQL score . | Day 100 . | 6 Months . | 1 Year . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| n . | Mean (SEM) . | P . | n . | Mean (SEM) . | P . | n . | Mean (SEM)* . | P . | |
| Parent proxy | |||||||||
| Self-Esteem | 21 | −15.12 (4.89) | .0057* | 20 | −11.46 (6.28) | .0839 | 15 | −3.61 (6.66) | .5959 |
| General Health Perception | 21 | 11.13 (2.51) | .0003* | 20 | 7.63 (3.31) | .0326 | 15 | 8.89 (5.27) | .1135 |
| Change in Health | 21 | 0.90 (0.38) | .0286 | 20 | 1.15 (0.36) | .0052* | 15 | 1.53 (0.48) | .0062* |
| Child | |||||||||
| Self-Esteem | 18 | −0.59 (4.51) | .8976 | 16 | −4.33 (5.87) | .4722 | 13 | 4.97 (5.33) | .3691 |
| General Health Perception | 18 | −1.90 (4.29) | .6631 | 16 | −6.69 (4.99) | .2001 | 13 | 6.99 (6.35) | .2927 |
| Change in Health | 18 | 0.33 (0.45) | .4691 | 16 | 0.19 (0.52) | .7225 | 13 | 1.46 (0.35) | .0013* |
Negative mean change denotes worsening HRQL score; positive mean change denotes improved HRQL score. Other items tested include the following (none of which reached the level of significance at any of the time points tested): physical functioning; social limitations due to emotional difficulties; social limitations due to behavioral difficulties; social limitations due to physical health; bodily pain and discomfort; behavioral; mental health; emotional impact; time impact; family activities; global health; global behavior; change in health; family cohesion; physical summary; and psychosocial summary.
SEM, standard error of the mean.
P values <.01 are statistically significant.
Discussion
Although EFS after HLA-matched sibling donor transplant is >90%, most patients do not have an HLA-matched sibling.4,5,21,37,38 This is the first multicenter URD transplant trial for SCD in North America and was conducted to expand access to transplantation utilizing HLA-matched URDs. The RIC regimen of alemtuzumab, melphalan, and fludarabine was used to overcome the higher risk of GR with URD BMT while limiting the toxicities associated with myeloablative regimens that may be exacerbated in patients with severe SCD and may limit patient acceptance.14,16,39,40
Although the trial met the prespecified 1-year end point of 75% EFS, the 1-year chronic GVHD rate was higher than expected after HLA-matched URD BMT, and GVHD was the predominant cause of death, noted primarily in older patients. Reports in African Americans with severe aplastic anemia (combining sibling and URD transplants) suggest an overall chronic GVHD rate of 36% (95% CI, 24-48) compared with 30% noted in whites (P = .36), although extensive chronic GVHD was observed more commonly in African Americans than in whites (72% vs 49%; P = .06), as was GVHD-related mortality.41 Other than race, additional factors may have influenced the observed high rates of chronic GVHD in this trial. HLA matching at HLA-DPB1 loci was not considered in donor selection, and a mismatch at this locus increases the risk of acute GVHD.42-44 Another plausible explanation could be the timing of alemtuzumab administration 3 weeks before infusion of the graft (distal administration) to overcome host rejection of the graft. It was timed to achieve low alemtuzumab levels at the time of graft infusion to maximize donor T-cell engraftment, and thus did not have a significant effect as a GVHD prophylaxis agent.21 Chronic GVHD rates are also expected to be higher in URD BMT and increase with recipient age; the higher rates observed in this trial are consistent with less protection against chronic GVHD.45-47 Further, the protocol recommended calcineurin inhibitor taper early (after day 100) in the absence of GVHD and may have contributed to de novo chronic GVHD subsequently. Chronic GVHD developed in 8 of 10 patients after they developed PRES symptoms. The protocol did not specify alternate GVHD prophylaxis in the event of PRES, and treatment was left to the center’s choice. Because it is common practice to discontinue or modify calcineurin inhibitor use after PRES, it is possible that the withdrawal or modification of the calcineurin inhibitor after the development of PRES may have additionally contributed to GVHD. Alemtuzumab or anti-human T-lymphocyte immune globulin used just proximal to transplant can offer better protection against GVHD but predisposes to mixed chimerism and rejection.48-50 Novel preparative agents such as treosulfan and GVHD prophylaxis methods such as posttransplant cyclophosphamide have shown recent promise in transplantation for SCD.51,52
HRQL improved significantly by 1 year posttransplant in the areas of Change in Health for these patients compared with pretransplant scores. Other HRQL domains did not show significant changes from baseline. The significant changes noted here, even in this small sample size, support that these children felt better and reported better functioning overall related to their health after transplant, despite the high incidence of chronic GVHD. We would expect patients with significant chronic GVHD after BMT to experience lower HRQL than those without, but the sample size was too small for a meaningful comparison between the two groups.
No patient developed hepatic sinusoidal syndrome or idiopathic pulmonary syndrome. However, one-third of the patients developed PRES (a known complication of SCD and hemoglobinopathy transplants) that was reversible, despite our recommendation for strict blood pressure monitoring and prompt intervention.53,54 Baseline blood pressure in SCD patients is generally lower than published norms for age, race, and sex, and the use of corticosteroid and calcineurin inhibitors may have exacerbated this complication. 53,55-57
In conclusion, the trial met its prespecified 1-year EFS, and significantly improved HRQL was reported posttransplant. However, although the RIC provided successful engraftment in the majority of patients, the regimen cannot be considered safe for widespread adoption without modification due to the RRT and high rate of chronic GVHD, which was the predominant cause of mortality. Future trials on URD transplantation for SCD should focus on strategies that minimize risks of GVHD and include stopping rules for chronic GVHD.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The Blood and Marrow Transplant Clinical Trials Network and the BMT CTN #0601 investigators gratefully acknowledge the contributions of the members of the External Review Committee: George Buchanan, University of Texas South Western, Dallas, TX; James Eckman, Emory University, Atlanta, GA; Alexis A. Thompson, Northwestern University Fienberg School of Medicine, Chicago, IL; and Catherine Wu, Dana Farber Cancer Institute, Boston, MA.
This work was supported by a grant from the National Institutes of Health, National Heart, Lung, and Blood Institute and National Cancer Institute (U10-HL069294) to the Blood and Marrow Transplant Clinical Trials Network, the National Marrow Donor Program, the Sickle Cell Disease Clinical Research Network, the National Center on Minority Health and Health Disparities, and the Pediatric Blood and Marrow Transplant Consortium.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the above-mentioned parties.
Authorship
Contribution: S.S., M.E., J.A.P., B.R.L., M.M.H., M.C.W., and N.K. designed the trial, interpreted the data, and drafted the manuscript; S.S., N.K., J.E.L., M.C.W., and M.E. adjudicated primary and secondary end points; J.W. prepared the data set and analyzed the data; J.A.P. and B.R.L. prepared and analyzed the HRQL data; all remaining authors critically reviewed the manuscript; and all authors approved the final manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Shalini Shenoy, Department of Pediatrics, Washington University School of Medicine, Box 8116, 660 S. Euclid Ave, St. Louis, MO 63110; e-mail: shenoy@wustl.edu.