Key Points
Subsets of ECs, including lymphatic and fenestrated ECs, but not conventional blood capillary ECs, synthesize FVIII.
von Willebrand factor and FVIII are coexpressed in postcapillary high endothelial venules but not in most other ECs.
Abstract
Circulating factor VIII (FVIII) is derived from liver and from extrahepatic sources probably of endothelial origin, but the vascular sites of FVIII production remain unclear. Among organs profiled, only liver and lymph nodes (LNs) show abundant expression of F8 messenger RNA (mRNA). Transcriptomic profiling of subsets of stromal cells, including endothelial cells (ECs) from mouse LNs and other tissues, showed that F8 mRNA is expressed by lymphatic ECs (LECs) but not by capillary ECs (capECs), fibroblastic reticular cells, or hematopoietic cells. Among blood ECs profiled, F8 expression was seen only in fenestrated ECs (liver sinusoidal and renal glomerular ECs) and some high endothelial venules. In contrast, von Willebrand factor mRNA was expressed in capECs but not in LECs; it was coexpressed with F8 mRNA in postcapillary high endothelial venules. Purified LECs and liver sinusoidal ECs but not capECs from LNs secrete active FVIII in culture, and human and mouse lymph contained substantial FVIII:C activity. Our results revealed localized vascular expression of FVIII and von Willebrand factor and identified LECs as a major cellular source of FVIII in extrahepatic tissues.
Introduction
Coagulation factor VIII (FVIII) is a pro-cofactor for factor IXa and circulates in tight complex with von Willebrand factor (vWF) at the stoichiometry of one FVIII molecule per 50 vWF monomers.1 Although vWF is synthesized by blood endothelial cells (ECs) and megakaryocytes, the cellular origins of FVIII have been controversial.2 Transplantation studies in dogs established that circulating FVIII is derived from extrahepatic tissues as well as from liver.2 Liver transplantation cured hemophilia3,4 ; however, transplantation of normal mesenteric lymph nodes (LNs) in dogs with hemophilia A corrected the FVIII level to about 20% of normal,5 and transplantation of lungs6 also corrected the defect. These studies suggested expression of FVIII by many cell types or by a widely distributed cellular source such as ECs. Consistent with an endothelial source, sinusoidal ECs, not hepatocytes, are the principal cell type in the liver that produce FVIII,7-9 and cultured human lung and glomerular microvascular ECs produce FVIII.10 But other studies have indicated that bone marrow hematopoietic stem cells and fibroblasts can also produce FVIII.11
Recent studies that used in vivo genetic approaches12,13 showed that FVIII production is restricted to vascular compartments; however, the FVIII-producing endothelial subtypes have not been characterized. Here we report that FVIII is produced by lymphatic ECs (LECs) and by subsets of postcapillary venule ECs and fenestrated ECs, but not by blood capillary ECs (capECs), nonendothelial fibroblastic reticular cells, or by hematopoietic cells from mouse LNs and other tissues. In contrast, the gene encoding vWF was highly expressed by capECs in many vessel beds, but not by LECs and glomerular ECs. Among ECs studied, only postcapillary high endothelial venules (HEVs), sites of active lymphocyte extravasation, clearly coexpressed both F8 and vWF. Our results define LECs as a major cellular source of extrahepatic FVIII and reveal discordant expression of FVIII and its carrier protein vWF.
Materials and methods
Isolation of subsets of endothelial cells and transcriptomic profiling
Six- to 8-week-old male and female BALB/c mice were used for EC isolation. These mice were bred and maintained in the animal facilities of the Veterans Affairs Palo Alto Health Care System, accredited by the Association for Assessment and Accreditation of Laboratory Animal Care. ECs were released from the surrounding stroma by enzymatic digestion while preserving their viability. The fluorescence-activated cell sorted EC subsets were placed directly into RNA lysis buffer, followed by total RNA isolation and microarray-based transcriptomic profiling as described.14 ECs were defined as CD31+ and as negative for lineage markers (CD45, CD11a, Ter119, and EPCAM) to eliminate potential contamination from hematopoietic cells and epithelial cells; LECs were defined as CD31+GP38+ (podoplanin); capECs were defined as CD31+GP38–MECA99+, which was validated by immunohistochemical staining15 ; HEVs were defined as CD31+GP38–MECA99– and as positive for either MAdCAM1 (MECA367+) or peripheral node addressin (PNAd; defined by antibody MECA79). We combined our transcriptomic profiling with data from the Immunological Genome Project (ImmGen Project)16 and from Nolan et al17 on the basis of the same normalization approach (GeneSpring preprocessing and default normalization [RMA-16] were applied). Antibodies were sourced as described.14 Raw expression above 150 units was considered significant.
FVIII:C activity assay using cell extracts, cell culture supernatant, and lymph
FVIII:C activity in cell lysates from fluorescence-activated cell sorted LECs or capECs and FVIII:C activity in culture supernatants or lymph were assayed as described9 by using the Biophen FVIII:C Kit (Aniara Diagnostica, West Chester, Ohio) according to the manufacturer’s instructions. Briefly, sorted cells were pelleted by centrifugation and resuspended in CelLytic M-cell lysis reagent (lysis buffer; Sigma-Aldrich) supplemented with a protease inhibitor cocktail (Roche), diluted 100-fold, incubated for 15 minutes on ice, and finally centrifuged for 5 minutes at 15 000g to remove the cell debris. The supernatant was then used for FVIII:C activity assay. The standard curve was generated by using recombinant FVIII formulated with sucrose (recombinant FVIII-FS) (Kogenate; Bayer) serially diluted from 20 mU/mL to 0.312 mU/mL in cell lysis buffer supplemented with protease inhibitors. For FVIII:C activity from culture supernatants, cells were cultured in 6-well petri dishes as described,18-20 and supernatants were collected 24 hours after cell confluence. The standard curve was generated by using recombinant FVIII-FS in the medium in which the cells were maintained. Mouse lymph was collected via cannulation of the thoracic duct21 of normal mice or from enlarged subcutaneous lymphatic sacs in mice injected subcutaneously with corn oil (100 μL) once per week for 4 to 6 weeks. The average volume of thoracic duct lymph collected from a mouse was ∼150 to 200 μL during 2 hours. Human afferent prenodal lymph samples were collected as described.22 The lymph was centrifuged at 1500g for 10 minutes to remove all cellular components. All lymph samples were clear at the time of collection, and contaminating hemoglobin levels in samples were measured as less than 1% of that in whole blood. For FVIII:C activity in mouse lymph, the standard curve was generated by using sodium citrated pooled normal mouse plasma (CD1; Bioreclamation IVT); for FVIII:C activity in human lymph, the standard curve was generated by using pooled normal human plasma (Factor Assay Control Plasma [FACT]; George King Bio-Medical, Inc.).
Results
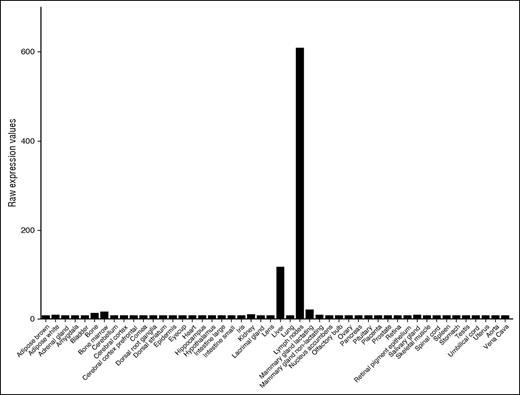
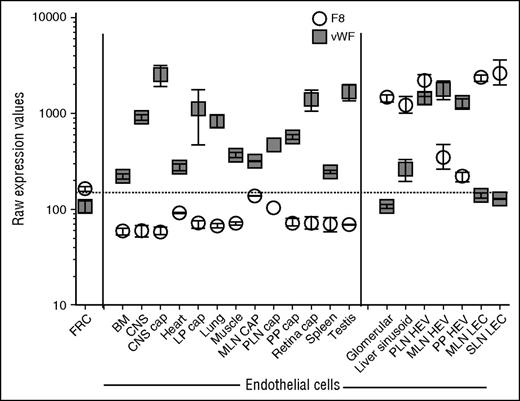
Survey of public databases (BioGPS and Geo Profiles) revealed that, among diverse tissues, F8 messenger RNA (mRNA) is most abundant in LNs and is more highly expressed in LNs than in the liver (Figure 1). To define the cellular origin of FVIII in LNs, we used a combination of cell-surface markers to purify HEVs and capECs from mouse LNs by fluorescence-activated cell sorting. Because our procedures were adapted from the published protocols of the ImmGen consortium, we were able to combine our data with ImmGen gene expression data on lymphoid tissue LECs, total blood endothelium, and other stromal and hematolymphoid populations. We also incorporated our transcriptomic profiles of capECs from brain, lamina propria of the intestine, Peyer’s patches, and retina, along with profiles of blood ECs from the bone marrow, brain, heart, kidney glomerulus, muscle, liver, lung, spleen, and testis.17 F8 mRNA is highly expressed in LECs, liver sinusoidal ECs (LSECs), and glomerular ECs (Figure 2); in contrast, it was not detectable in fibroblastic reticular stromal cells, skin fibroblasts, thymic medullary stromal cells, or hematopoietic cells (data not shown). LECs also expressed genes encoding the FVIII escort proteins LMAN1 and MCFD2, necessary for efficiently transporting FVIII protein from the endoplasmic reticulum to the endoplasmic reticulum-Golgi intermediate compartment23 (data not shown). HEVs, vessels specialized for recruiting lymphocytes from the blood, also expressed F8: F8 mRNA was expressed in HEVs from LNs (Figure 2) and at low but significant levels in HEVs from Peyer’s patches. Notably, F8 was not detectably expressed in blood ECs or capECs from bone marrow, brain, heart, lamina propria of intestines, LNs, lung, muscle, Peyer’s patches, retina, or testis. Extensive searches of publicly available microarray databases (ImmGen Project and Geo Profiles) failed to find significant levels of F8 mRNA expression in profiled hematopoietic cells, including monocytes and hematopoietic stem cells; epithelial cells from various tissues; endothelial cells from large arteries including aortic, coronary, and iliac arteries; or from large veins such as pulmonary and iliac veins (ImmGen and Geo Profiles).17,24,25
Expression of F8 mRNA in various mouse tissues. The data are from BioGPS and Geo Profiles and are presented as raw expression values.
Expression of F8 mRNA in various mouse tissues. The data are from BioGPS and Geo Profiles and are presented as raw expression values.
Expression of F8 and vWF mRNA in fluorescence-activated cell sorted subtypes of ECs from various tissues. Lymphoid tissue ECs were released from the surrounding stroma by enzymatic digestion while preserving their viability, and the sorted EC subsets were then transcriptomically profiled. The transcriptomes of the sorted EC subtypes were combined with data from sorted ECs16,17 analyzed with the same microarray platform (Affymetrix Mouse Gene 1.0 ST arrays) and normalized on the basis of GeneSpring preprocessing and default normalization (RMA-16). The levels of F8 and vWF mRNA were plotted from these normalized transcriptomes. Raw Affymetrix expression values of at least 3 biological replicates (except n = 1 for PLN CAP; some values were excluded because of contamination with HEV-specific genes). Mean ± standard error of the mean. BM, bone marrow; cap, capillary; CNS, central nervous system; FRC, fibroblastic reticular cell; LP, lamina propria; MLN, mesenteric lymph node; PLN, peripheral lymph node; PP, Peyer’s patches SLN, subcutaneous lymph node.
Expression of F8 and vWF mRNA in fluorescence-activated cell sorted subtypes of ECs from various tissues. Lymphoid tissue ECs were released from the surrounding stroma by enzymatic digestion while preserving their viability, and the sorted EC subsets were then transcriptomically profiled. The transcriptomes of the sorted EC subtypes were combined with data from sorted ECs16,17 analyzed with the same microarray platform (Affymetrix Mouse Gene 1.0 ST arrays) and normalized on the basis of GeneSpring preprocessing and default normalization (RMA-16). The levels of F8 and vWF mRNA were plotted from these normalized transcriptomes. Raw Affymetrix expression values of at least 3 biological replicates (except n = 1 for PLN CAP; some values were excluded because of contamination with HEV-specific genes). Mean ± standard error of the mean. BM, bone marrow; cap, capillary; CNS, central nervous system; FRC, fibroblastic reticular cell; LP, lamina propria; MLN, mesenteric lymph node; PLN, peripheral lymph node; PP, Peyer’s patches SLN, subcutaneous lymph node.
vWF serves as a plasma-carrier protein for FVIII, prolonging the half-life of FVIII in blood.26 vWF also mediates platelet adhesion to damaged vascular subendothelium and is important for the formation of a hemostatic plug in vessels under high fluid shear stress.27 Circulating vWF is produced by the vascular endothelium, with a minor contribution from platelets and/or megakaryocytes. In blood ECs, vWF is synthesized and stored in Weibel-Palade bodies.28 vWF and F8 mRNA expression are poorly correlated except in HEVs (Figure 2). In contrast to F8, vWF is highly expressed in capECs from multiple tissues and in HEVs from LNs and Peyer’s patches, yet at a lower but significant level in LSECs, and minimally if at all in the glomerular EC and LEC sites of F8 expression. This divergent expression of vWF and F8 mRNA was surprising but is consistent with the existence of both constitutive sources and desmopressin (DDAVP)-releasable pools of FVIII in vivo. Desmopressin is a pharmacologic agent that induces degranulation of vWF-containing Weibel-Palade bodies and elevates circulating levels of vWF and FVIII in patients with moderate hemophilia A.29 Because coproduction of vWF and FVIII targets FVIII into storage granules,30 the desmopressin-inducible source of FVIII is likely to be from the storage granules of ECs coexpressing vWF and FVIII. The poor expression of vWF in LSECs suggests that LSECs might secrete FVIII constitutively, which is consistent with the lack of desmopressin-releasable FVIII response in men with hemophilia A after liver transplantation.31 Coexpression of F8 and vWF in HEVs suggests that HEVs, and potentially other postcapillary venules, may be a source of desmopressin-releasable FVIII. Rapid release of preformed vWF-FVIII complexes could facilitate local hemostasis at sites of active lymphocyte transendothelial migration, plugging holes to prevent blood leakage. Conversely, generalized EC coexpression of FVIII and vWF might pose a thrombotic risk, as observed with the release of ultralarge vWF multimers upon cellular stimuli, particularly in the absence of ADAMTS13.32 The separation of FVIII and vWF production may be necessary to avoid local thrombosis at sites such as brain capECs.
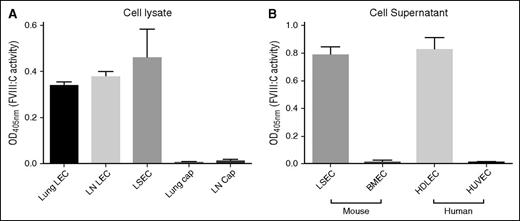
To determine whether LECs produce biologically active FVIII, we isolated mouse LECs and capECs by fluorescence-activated cell sorting from lymph nodes and lung and assayed FVIII:C activity in lysates of the purified cells by using purified LSECs for comparison. FVIII:C was detected in LEC lysates at levels comparable to those in purified LSECs on the basis of an equal number of cells (Figure 3A). In contrast, no FVIII:C activity was detected in capEC lysates from lung or brain. We also assayed supernatants from cultured cells: FVIII:C activity was readily detected in supernatants from primary cultured mouse LSECs and human dermal LECs, but not from mouse brain capECs or human umbilical vein endothelial cells (Figure 3B). The biological activity of FVIII in these cells correlates well with the levels of F8 mRNA. We detected FVIII:C and F8 mRNA in lung LECs rather than in lung capECs, suggesting that FVIII:C activity in lung microvascular ECs reported in a prior study10 is principally derived from LECs.
Biologically active FVIII is present in cell lysates or culture supernatants of purified LECs and LSECs, but not in those of conventional capECs. FVIII activity was measured by using a Biophen FVIII:C Kit and was normalized to equal cell numbers. (A) Biological activity of FVIII in cell lysates of purified LECs and LSECs. Mean ± standard deviation of 3 biological replicates except lung LECs and lung capECs that were the average of 2 experiments. (B) FVIII activity in supernatants (24 hours of cell culture) was detected from primary cultured LSECs and human dermal LECs (HDLECs), but not from primary brain microvascular ECs (BMECs) and human umbilical vein ECs (HUVECs). The activity was normalized for cell number. The data are from 3 biological replicates.
Biologically active FVIII is present in cell lysates or culture supernatants of purified LECs and LSECs, but not in those of conventional capECs. FVIII activity was measured by using a Biophen FVIII:C Kit and was normalized to equal cell numbers. (A) Biological activity of FVIII in cell lysates of purified LECs and LSECs. Mean ± standard deviation of 3 biological replicates except lung LECs and lung capECs that were the average of 2 experiments. (B) FVIII activity in supernatants (24 hours of cell culture) was detected from primary cultured LSECs and human dermal LECs (HDLECs), but not from primary brain microvascular ECs (BMECs) and human umbilical vein ECs (HUVECs). The activity was normalized for cell number. The data are from 3 biological replicates.
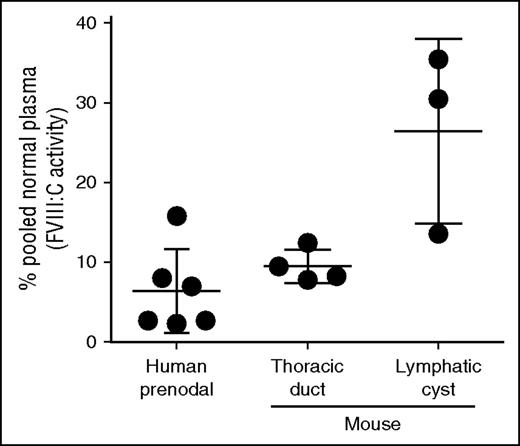
If LECs produce FVIII, then lymph would be expected to contain FVIII. To test this, we collected normal mouse lymph by cannulation of the thoracic duct from 4 mice and human afferent prenodal lymph from 6 healthy volunteers. The FVIII levels in mouse lymph ranged from 8% to 12% (mean, 9.5% ± 2.1% standard deviation) and in human lymph from 3% to 16% (mean, 6.4% ± 5.2% standard deviation) of measured pooled plasma levels in normal mice and humans, respectively (Figure 4). Similar values were reported for rabbit limb lymph.33 We found higher concentrations of FVIII, up to 30% of the plasma level, in lymph from lymphatic cysts. The higher and more variable FVIII level in mouse afferent lymphatic cysts may be the result of accumulation of lymph through local production in the absence of lymph flow. Proteomic analysis of human afferent prenodal lymph confirmed the presence of FVIII-derived peptides with false discovery rate <0.004% (data not shown).
Substantial amounts of biologically active FVIII are present in human prenodal and mouse thoracic or cyst lymph. Lymph was collected as described in the text, and FVIII:C activity was measured by using the Biophen FVIII:C Kit. The data represent FVIII:C activities of individual lymph samples relative to that of human or mouse pooled normal plasma level of FVIII (defined as 100%) as described in “Materials and methods.”
Substantial amounts of biologically active FVIII are present in human prenodal and mouse thoracic or cyst lymph. Lymph was collected as described in the text, and FVIII:C activity was measured by using the Biophen FVIII:C Kit. The data represent FVIII:C activities of individual lymph samples relative to that of human or mouse pooled normal plasma level of FVIII (defined as 100%) as described in “Materials and methods.”
The half-life of FVIII in plasma is estimated to be 9 to 18 hours (estimated average, 12 hours),34-36 suggesting that approximately three-fourths of the FVIII in plasma must be replenished every day. An estimated 3 L of lymph is added to the blood each day, approximately equivalent to 110% of the volume of plasma. Because lymph contains, on average, ∼5% to 10% of blood plasma FVIII levels, the steady flow of lymph would contribute ∼8% to ∼15% of newly synthesized FVIII per day (FVIII in lymph entering the blood per day/FVIII replenished in blood per day = (3 L lymph × 5% to 10% × [plasma FVIII])/(0.75 × 2.75 L plasma × [plasma FVIII]) = 7.5% to 15%). Consistent with a significant contribution from lymph, chronic lymphatic fistulae are associated with reduced FVIII levels and overt hemostatic defects.37 We may under- or overestimate the lymphatic contribution of FVIII, because lymph flow varies significantly with fluid intake,38 body movement,39 and anatomic location.40 Interestingly, prior studies showed an increase in FVIII after exercise.41,42 Genetic approaches to delete F8 specifically in LECs will further elucidate the lymphatic EC contribution to FVIII levels.
Discussion
We conclude that subtypes of ECs, including LECs, fenestrated ECs, and some venules (at sites of leukocyte traffic) but not conventional blood capECs, synthesize FVIII and that vWF and FVIII production are most often dissociated among EC subsets. Localized FVIII production may be a critical determinant of hemostasis, acting in conjunction with circulating FVIII-vWF complexes. All FVIII-producing EC subtypes are loosely surrounded by neighboring cells: LSECs by liver stellate cells,43 glomerular ECs by podocytes and mesangial cells,44 HEVs by fibroblastic reticular cells,45 and LECs by a basement membrane anchored via filaments to the surrounding tissues.46 The specialized vessel beds may account for EC-subtype-specific expression of FVIII. The identification of these FVIII-producing EC subtypes makes it possible to elucidate the genetic determinants that regulate FVIII synthesis. Moreover, LECs can be cultured or generated from induced pluripotent stem cells47,48 and are accessible for therapeutic manipulation and transplantation49 : they may serve as an ideal cell source or target for cell- or gene-based FVIII therapy for hemophilia A.
During the course of preparing this article, an interesting study on F8 expression in human ECs was published by Turner and Moake.50 Our finding of F8 expression in mouse kidney glomerular ECs is consistent with their findings in human glomerular ECs. Although we did not detect a significant amount of FVIII:C activity in the supernatant from cultured HUVECs, we detected a significant amount of intracellular FVIII protein in HUVECs in a flow cytometry study (data not shown) using mouse monoclonal anti-human FVIII antibody (LS-B3124), which is consistent with their finding that FVIII is costored with vWF in the Weibel-Palade bodies of HUVECs.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Lawrence Leung and Qingyu Wu for their support.
This work was supported by National Institutes of Health (NIH) grant nos. R37 AI047822, AI093981, and U19 AI090019 from the National Institute of Allergy and Infectious Diseases, grant no. R01 GM37734 from the National Institute of General Medical Sciences, an award from the Department of Veterans Affairs (E.C.B.), a seed grant from the Stanford Cardiovascular Institute (E.C.B. and J.P.), a Biogen research grant (J.P. and E.C.B.), an Immunity, Transplantation and Infection Young Investigator Award (C.J.C.), a Fellowship Award from the Ludwig Edinger Foundation (C.J.C), fellowship awards from the Crohn’s and Colitis Foundation (M.L.) and from the American Heart Association (H.K.), and a Stanford Cardiovascular Institute award (T.T.D.). T.T.D. was the recipient of a fellowship under NIH training grant nos. T32 AI07290 from the NIAID and T32 HL098049 from the National Heart, Lung, and Blood Institute. A.R. was supported in part by an internship under California Institute for Regenerative Medicine grant no. TB1-01195. L.Z. was supported by a Novo Nordisk Haemophilia Research Fund in China.
Authorship
Contribution: J.P. designed and conducted research, analyzed data, and wrote the manuscript; E.C.B. designed research, analyzed data, and wrote the manuscript; J.M. helped to analyze data and write the manuscript; M.L., A.S., C.J.C., H.K., T.T.D., and A.R. conducted research and analyzed data; and L.Z., L.X., H.J., and L.S. provided vital reagents and analyzed data.
Conflict-of-interest disclosure: H.J. is an employee of Biogen. The remaining authors declare no competing financial interests.
Correspondence: Junliang Pan, Stanford University School of Medicine, 3801 Miranda Ave, Building 101, Room C4-101, Palo Alto, CA 94304; e-mail: jpan@stanford.edu.