Abstract
Sickle cell disease (SCD) is a severe genetic blood disorder characterized by hemolytic anemia, episodic vaso-occlusion, and progressive organ damage. Current management of the disease remains symptomatic or preventative. Specific treatment targeting major complications such as vaso-occlusion is still lacking. Recent studies have identified various cellular and molecular factors that contribute to the pathophysiology of SCD. Here, we review the role of these elements and discuss the opportunities for therapeutic intervention.
Introduction
Sickle cell disease (SCD) originates from a single nucleotide mutation of β-globin, which leads to polymerization of the mutated hemoglobin (Hb) upon deoxygenation, and dramatic alteration in the shape and surface properties of red blood cells (RBCs). Through interactions with multiple blood and immune cell populations, sickle RBCs promote inflammation, obstruct the vasculature, and injure the endothelium, leading to broad manifestations that affect most vital organs.1-4 Although the molecular origin of the disease is clear, the mechanisms that contribute to the complex manifestation and severe outcome of the disease have not been fully elucidated. Recent studies have revealed that many cell types that are not affected by the β-globin mutation play important roles in the pathophysiology of SCD,5 leading to an evolving multicellular paradigm that has triggered enthusiastic investigations into novel therapeutics for the disease.
Neutrophils in SCD
Neutrophils are a critical component of innate immunity. Being the most abundant immune cells in the circulation, they provide immune protection against invading pathogens but can also promote certain inflammatory diseases.6,7 Neutrophils are initially suggested to promote disease progression in SCD by clinical epidemiological studies. SCD patients were found to exhibit marked variation in disease severity. For example, in patients with painful crises, the most common disease manifestation, the rates of crises vary from 0 to >10 episodes per year.8,9 Notably, patients with more severe clinical manifestations tend to have higher neutrophil counts compared with racially matched controls.10 High leukocyte counts also positively correlate with early death, silent brain infarcts, hemorrhagic strokes, and acute chest syndrome (ACS) in SCD patients,11-14 implicating leukocyte count (neutrophil in particular) as a major risk factor for SCD.
Further evidence supporting a role for neutrophils in SCD pathophysiology comes from the identification of myeloid growth factors, ie, granulocyte macrophage colony-stimulating factor (GM-CSF) and granulocyte colony-stimulating factor (G-CSF), as absolute contraindications in SCD individuals. In early reports, severe or fatal crises have occurred in SCD patients administered with either GM-CSF or G-CSF to treat leg ulcer, mobilize hematopoietic stem cells, or correct neutropenia.15-18 More recently, a patient was reported to have a rare co-existence of SCD and severe congenital neutropenia, exhibiting significantly alleviated disease manifestations compared with his siblings. However, when the patient received G-CSF to treat neutropenia, the course of the disease dramatically worsened.19
By contrast, a reduction in neutrophil count can benefit SCD. In a multicenter study of hydroxyurea, hydroxyurea treatment (ie, the most commonly used therapeutics for SCD patients) markedly decreased the frequency of painful crises and ACS in patients with moderate to severe SCD.20 Hydroxyurea has been shown to effectively induce fetal Hb (HbF) expression in RBCs, but it has also many other effects that benefit SCD.21-24 For example, hydroxyurea treatment significantly decreases soluble vascular cell adhesion molecule (VCAM)-1 levels in patient plasma and reduces the adhesion of sickle RBCs to the endothelium.22,23 In addition, recent studies also suggest that hydroxyurea treatment increases nitric oxide (NO) species, which may or may not be associated with induction of HbF.21,25-27 Interestingly, hydroxyurea treatment shows beneficial effects even in patients with no detectable rise of HbF, whereas all patients who respond well clinically to hydroxyurea treatment have decreased numbers of neutrophils.22,28,29
Neutrophils from patients with SCD also exhibit an activation phenotype characterized by a lower expression level of l-selectin (CD62L) and a higher level of CD64.30 In addition, CD11b/CD18 membrane expression is also ∼70% higher on neutrophils from SCD patients compared with controls.31 These neutrophils show increased adhesive properties, which could be reduced by stimulation of the NO/cyclic guanosine monophosphate (cGMP)-dependent pathways.32 Hydroxyurea treatment is found to suppress neutrophil activation as demonstrated by the correction of neutrophil activation markers.33 Further studies suggest that hydroxyurea treatment has immediate benefits on sickle cell vaso-occlusion by inhibiting neutrophil recruitment and activation, with a mechanism that involves the amplification of the NO-cGMP pathway.25,26 These findings suggest an important role of neutrophils in the pathophysiology of SCD.
Neutrophil-RBC interactions promote vaso-occlusion
The first evidence that neutrophils may directly participate in the pathogenesis of SCD comes from the observation that sickle RBCs bind to neutrophils in vitro.3 This observation is supported by in vivo studies in SCD mice (Berkeley mice34 ), where the dynamics of circulating blood cells are analyzed in the cremasteric microcirculation using intravital microscopy.35 In this model, sickle RBCs are found to predominantly interact with adherent leukocytes in postcapillary venules. These interactions are triggered by surgical trauma, and enhanced and sustained by tumor necrosis factor (TNF)-α administration, leading to a lethal vaso-occlusive crisis (VOC). Mice lacking both P- and E-selectins, in which leukocytes are prevented from recruitment to the endothelium, are protected from VOC in this model.35
Further studies using multichannel fluorescence intravital microscopy identify Gr-1+ neutrophils as the major leukocyte population that is recruited to postcapillary venules and interacts with circulating RBCs in TNF-α stimulated mice.36 Sickle RBCs are captured by activated αMβ2 integrin (macrophage-1 antigen [Mac-1]) clustered on the leading edge of adherent neutrophils, leading to acute lethal VOC.37 The activation of Mac-1 on adherent neutrophils requires a secondary wave of signals transduced by E-selectin ligand-1 upon engagement of neutrophils by endothelial E-selectin. Inactivation of E-selectin and Mac-1 by either genetic manipulation or antibody blocking prevents neutrophil-RBC interactions, leading to improved blood flow and prolonged survival of SCD mice.37
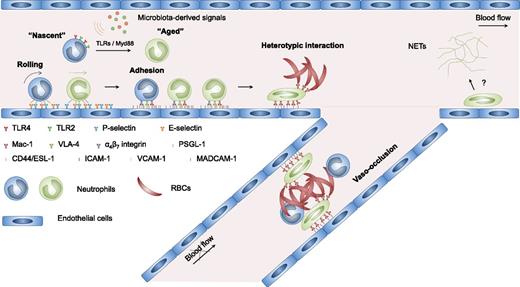
These studies suggest that vaso-occlusion in SCD may arise from a multistep and multicellular process as detailed in Figure 1. First, endothelial cells are activated by internal and exogenous inflammatory stimuli, enabling the recruitment of circulating neutrophils to postcapillary venules. Then adherent neutrophils interact with circulating sickle RBCs through activated Mac-1, leading to transient or prolonged obstruction of venular blood flow. The obstruction of blood flow increases the transit time of RBCs, produces ischemia, enhances RBC sickling, and further promotes neutrophil recruitment and heterotypic interactions, all of which culminate in vascular occlusions that may cause life-threatening crises.
Multicellular and multistep model of sickle cell vaso-occlusion. Sickle cell vaso-occlusion arises from a cascade of interactions among RBCs, neutrophils, and endothelial cells. Activation of endothelial cells leads to the recruitment of neutrophils, which is initiated by rolling of neutrophils on endothelial selectins, followed by adhesion mediated by integrins. Adherent neutrophils receive a secondary wave of signals transduced through E-selectin, leading to the activation of αMβ2 (Mac-1) integrin on the leading edge. Activated Mac-1 on adherent neutrophils mediates the capture of circulating sickle RBCs, producing a temporary or prolonged obstruction of venular blood flow. Circulating neutrophils exhibit considerable heterogeneity in their proinflammatory properties. Signals derived from the microbiota drive the neutrophil aging in the circulation, generating an overly active aged subset that exhibits enhanced Mac-1 activation and NETs formation. Aged neutrophils play an important role in promoting sickle cell vaso-occlusion. Currently, whether NET formation plays a role in the vaso-occlusive process remains unclear. ESL-1, E-selectin ligand-1; MADCAM-1, mucosal vascular addressin cell adhesion molecule-1; PSGL-1, P-selectin glycoprotein ligand-1; VLA-4, very late antigen-4.
Multicellular and multistep model of sickle cell vaso-occlusion. Sickle cell vaso-occlusion arises from a cascade of interactions among RBCs, neutrophils, and endothelial cells. Activation of endothelial cells leads to the recruitment of neutrophils, which is initiated by rolling of neutrophils on endothelial selectins, followed by adhesion mediated by integrins. Adherent neutrophils receive a secondary wave of signals transduced through E-selectin, leading to the activation of αMβ2 (Mac-1) integrin on the leading edge. Activated Mac-1 on adherent neutrophils mediates the capture of circulating sickle RBCs, producing a temporary or prolonged obstruction of venular blood flow. Circulating neutrophils exhibit considerable heterogeneity in their proinflammatory properties. Signals derived from the microbiota drive the neutrophil aging in the circulation, generating an overly active aged subset that exhibits enhanced Mac-1 activation and NETs formation. Aged neutrophils play an important role in promoting sickle cell vaso-occlusion. Currently, whether NET formation plays a role in the vaso-occlusive process remains unclear. ESL-1, E-selectin ligand-1; MADCAM-1, mucosal vascular addressin cell adhesion molecule-1; PSGL-1, P-selectin glycoprotein ligand-1; VLA-4, very late antigen-4.
Neutrophil heterogeneity and microbiota regulation
Circulating neutrophils have long been considered a homogenous population. However, heterogeneity may arise from their aging in the circulation and replenishment by newly released neutrophils from the bone marrow.38 Recent studies show that aged neutrophils, marked by CD62Llo CXCR4hi, represent an overly active neutrophil subset that exhibits enhanced Mac-1 activation and neutrophil extracellular traps (NETs) formation under inflammatory conditions. Interestingly, neutrophil aging is driven by microbiota-derived signals through neutrophil toll-like receptors (TLRs) and Myd88-mediated signaling (Figure 1). Microbiota depletion or genetic deficiency of these signaling pathways leads to reduced aged neutrophil numbers and diminished Mac-1 activation in the vasculature. In SCD mice, the aged neutrophil population is dramatically expanded, and the increased aged neutrophil counts correlate positively with neutrophil adhesion, Mac-1 activation, and neutrophil-RBC interactions. Microbiota depletion using broad spectrum antibiotics largely normalizes the numbers of aged neutrophils in SCD mice and markedly reduces neutrophil adhesion, Mac-1 activation, and neutrophil-RBC interactions, leading to significantly improved blood flow and prolonged survival. In addition, chronic depletion of the microbiota protects SCD mice from tissue damage. For example, splenomegaly and liver damage induced by SCD are significantly alleviated in antibiotics-treated SCD mice.39
Patients with SCD have also been analyzed for aged neutrophil numbers.39 Consistent with the rodent data, the numbers of aged neutrophils are dramatically increased in SCD patients compared with healthy controls. Strikingly, in the patient population taking penicillin prophylaxis, the numbers of aged neutrophils are significantly reduced.39 Although penicillin V can clearly prevent morbidity and mortality from Streptococcus pneumoniae infection in young children with SCD,40,41 whether antibiotics can mitigate vaso-occlusive episodes remains unknown. These results thus raise the possibility that antibiotics therapy may have a broader impact in the management of the disease, which should be evaluated in clinical trials.
Platelets in SCD
Platelets are essential for hemostasis but can also promote inflammation.42 Platelet activation is elevated in SCD patients under steady-state conditions, which is further enhanced during VOC.43-45 Activated platelets may promote the adhesion of sickle RBCs to human vascular endothelium by secreting thrombospondin,46 and may contribute to thrombosis and pulmonary hypertension in SCD.43 Platelets can also bind to erythrocytes, monocytes, and neutrophils to form aggregates.45,47 Significantly more platelet-monocyte aggregates and platelet-neutrophil aggregates are observed in the circulation of both SCD patients and transgenic SCD mice compared with controls.48 Activated platelets bind to neutrophils to form aggregates in a P-selectin–dependent manner.49 Pulmonary dysfunction is significantly ameliorated in SCD mice when platelet-neutrophil aggregate formation is blocked by P-selectin neutralizing antibody or the platelet inhibitor clopidogrel.49 At the molecular level, the serine/threonine kinase isoform AKT2 in neutrophils appears essential for neutrophil crawling and neutrophil-platelet interactions on activated endothelial cells during vascular inflammation.50 Inhibition of AKT2 diminishes neutrophil adhesion and neutrophil-platelet interactions, leading to improved blood flow in SCD mice.50
Platelets can also be captured by activated Mac-1 integrin clustered on the leading edge of adherent neutrophils, promoting vascular damage and lung injury by generating oxidative species in a mouse model of transfusion-related acute lung injury.37 In addition, platelet interaction can also induce neutrophil polarization through P-selectin glycoprotein ligand-1 transduced signals, triggering directed migration of neutrophils to initiate inflammation.51 Although these studies indicate a role for platelets in the pathogenesis of SCD, direct evidence that platelets participate in major complications such as vaso-occlusion in SCD is still lacking. Our own studies have found surprisingly little platelet accumulation at sites of active VOC (P.S.F., unpublished data).
Hemolysis, NETs, and acute lung injury
Hemolysis in SCD arises from damaged sickle RBC membranes, causing chronic anemia and the release of Hb into the circulation, which promotes inflammation by depleting NO, generating oxidative stress, and releasing heme, the prosthetic moiety of Hb.52 A large fraction of extracellular heme is carried by the erythrocyte-derived microparticles in circulation.53 These heme-loaded microparticles transfer heme to endothelial cells, leading to vascular dysfunction and vaso-occlusion in SCD.53 Activation by extracellular heme increases expression of adhesion molecules on endothelial cells, thus promoting leukocyte recruitment.54 Heme can also contribute to the pathogenesis of SCD by activating placenta growth factor (PlGF) production from erythroid cells via erythroid Krüppel-like factor.55
Recent studies have found that plasma heme levels are elevated during VOC in both human patients and SCD mice, leading to neutrophil activation and the formation of NETs in pulmonary vaculature.56 NETs in pulmonary vasculature contribute to acute lung injury in SCD mice challenged with TNF-α, which is significantly alleviated by administration of DNAse I or hemopexin, the reagent that dismantles NETs or scavenges heme, respectively.56 The administration of exogenous heme or hemin, the oxidized form of heme, can trigger VOC or ACS in SCD mice through activation of the endothelium, which is largely prevented by TLR4 inhibition, suggesting that extracellular heme/hemin may signal through TLR4 to trigger an inflammatory response.57,58 In addition, hemolytic transfusion reactions (HTRs) induce lethal acute vaso-occlusion in a model of alloimmune, immunoglobulin-mediated HTRs in SCD mice.59 These studies suggest that VOC and acute lung injury may be interconnected by neutrophils, NETs, and hemolysis.
Platelets can also activate neutrophils to produce NETs. During bacterial infection, platelets are activated by TLR4 ligand in the circulation and then bind to the adherent neutrophils, leading to the formation of NETs in liver sinusoids and pulmonary capillaries.60 Platelets also promote NET formation in transfusion-related acute lung injury.61 Targeting platelet activation with aspirin or a glycoprotein IIB/IIIA inhibitor reduces NET formation and alleviates lung injury.61 Similarly, aspirin inhibits neutrophil-platelet aggregate formation and thereby protects the lungs in an acute lung injury model.62 These studies raise the possibility that platelets may contribute to SCD pathophysiology by promoting NET formation, although direct evidence is still needed.
Inflammation in SCD
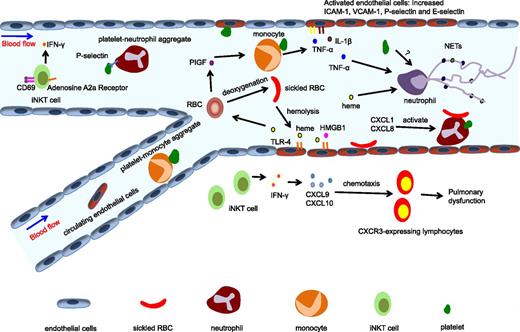
SCD has long been recognized as a chronic inflammatory disease. Accumulating evidence reveals that cell types not affected by the mutation, such as leukocytes and endothelial cells, play important roles in SCD pathophysiology. In addition, the list of molecules that promote inflammation in SCD is also expanding (Figure 2).
Inflammation in SCD. SCD has been recognized as a chronic inflammatory disease. Multiple cell types and molecules are involved in its inflammatory pathways. Hemolysis of RBCs leads to the release of heme into the circulation, which can activate endothelial cells through the TLR4 pathway and induce PlGF release from RBCs. PlGF activates monocytes, leading to the production of proinflammatory cytokines TNF-α and IL-1β. The adhesion of platelets or sickle RBCs can also activate endothelial cells, producing increased expression of adhesion molecules including ICAM-1, VCAM-1, P-selectin, and E-selectin, and thus promoting the recruitment of neutrophils. iNKT cells exhibit an activated phenotype and contribute to pulmonary dysfunction in SCD by producing IFN-γ and inducing CXCR3 chemokines. Platelets can form aggregates with circulating leukocytes, including neutrophils and monocytes. Activated neutrophils roll and adhere to the endothelium, and initiate VOC by capturing sickle RBCs. Heme can also induce NET formation, which promotes acute pulmonary injury in SCD. HMGB1, high mobility group box 1; iNKT cell, invariant natural killer T cell.
Inflammation in SCD. SCD has been recognized as a chronic inflammatory disease. Multiple cell types and molecules are involved in its inflammatory pathways. Hemolysis of RBCs leads to the release of heme into the circulation, which can activate endothelial cells through the TLR4 pathway and induce PlGF release from RBCs. PlGF activates monocytes, leading to the production of proinflammatory cytokines TNF-α and IL-1β. The adhesion of platelets or sickle RBCs can also activate endothelial cells, producing increased expression of adhesion molecules including ICAM-1, VCAM-1, P-selectin, and E-selectin, and thus promoting the recruitment of neutrophils. iNKT cells exhibit an activated phenotype and contribute to pulmonary dysfunction in SCD by producing IFN-γ and inducing CXCR3 chemokines. Platelets can form aggregates with circulating leukocytes, including neutrophils and monocytes. Activated neutrophils roll and adhere to the endothelium, and initiate VOC by capturing sickle RBCs. Heme can also induce NET formation, which promotes acute pulmonary injury in SCD. HMGB1, high mobility group box 1; iNKT cell, invariant natural killer T cell.
Cells that promote inflammation in SCD
Monocytes exhibit an activated phenotype and promote inflammation in SCD.63-65 Monocytes from SCD patients can activate the nuclear factor κB (NF-κB) pathway in endothelial cells. The activation of the NF-κB pathway results in higher expression of adhesion molecules, including E-selectin, intercellular adhesion molecule (ICAM)-1, and VCAM-1, thus promoting mononuclear leukocytes adhesion to endothelial cells. The activation of endothelial cells by monocytes does not require cell-cell contact, but is mediated by proinflammatory cytokines produced by monocytes. When TNF-α and interleukin (IL)-1β from monocytes are blocked with neutralizing antibodies, the upregulation of E-selectin expression by endothelial cells is abrogated.63 Importantly, monocytes isolated from SCD patients have higher TNF-α and IL-1β expression levels compared with healthy controls.64 Although the mechanisms that contribute to monocyte activation in SCD remain largely unclear, recent studies have identified several molecular and cellular factors important for this phenomenon. The first clue has emerged from the studies showing that platelet binding to monocytes leads to monocyte activation.66 The percentage of platelet-monocyte aggregates is significantly higher in SCD patients compared with healthy controls, suggesting an increased activation level of monocytes.66 The elevated levels of PlGF in SCD patients also contribute to monocytes activation.67,68 PlGF, an angiogenic growth factor belonging to the vascular endothelial growth factor family, is produced by erythroid cells. Clinical studies found that PlGF levels positively correlate with the severity of disease manifestation in patients with SCD.68,69 PlGF significantly increases the expression of proinflammatory cytokines including IL-1β, TNF-α, IL-8, monocyte chemoattractant protein-1, and vascular endothelial growth factor via the activation of Flt-1, and the PI3K/AKT and ERK-1/2 pathways in peripheral blood monocytes.67
iNKT cells have been shown to play a key role in promoting pulmonary inflammation and dysfunction in SCD.70,71 iNKT cells are a subset of T cells that have limited T-cell receptor repertoire and recognize lipid antigens presented by CD1d on antigen-presenting cells. iNKT cells are thought to bridge innate and adaptive immunity by producing large amounts of cytokines shortly after stimulation.72 In NY1DD transgenic SCD mice, more numerous and activated iNKT cells (CD69+ IFN-γ+) that are hypersensitive to hypoxia/reoxygenation are found in the spleen, liver, and lung compared with controls.70 iNKT cells cause baseline pulmonary dysfunction by producing IFN-γ and IFN-γ–induced CXCR3 chemokines in SCD mice, which can be prevented by genetic deficiency or pharmacologic inactivation of iNKT cells.70 Further studies have found that iNKT cells in SCD mice also exhibit higher expression levels of the adenosine 2A receptor (A2AR), a receptor that triggers anti-inflammatory response upon activation. Treatment of SCD mice with A2AR agonist regadenoson could effectively prevent baseline pulmonary dysfunction in SCD mice.73 Importantly, SCD patients also exhibit more numerous and activated iNKT cells in the circulation.70 During painful VOC, iNKT cells become more activated (CD69+ IFN-γ+ IL-4+), and express higher levels of A2AR in an NF-κB–dependent manner in SCD patients.74,75 These results suggest that targeting iNKT cells by either humanized monoclonal antibodies (mAbs) or A2AR agonist may benefit SCD.
Endothelial cells have been described to have an activated phenotype in SCD.66 The activation of endothelial cells may be induced by adhesion of sickle RBCs, which produces oxidative stress, activates the NF-κB pathway, and promotes the transmigration of monocytes.2 Treatment with an NF-κB pathway inhibitor reverses the activation phenotype of circulating endothelial cells in SCD patients, and of both circulating and tissue endothelial cells in SCD transgenic mice in vivo.76 Furthermore, monocytes-derived TNF-α and IL-1β also activate endothelial cells via the NF-κB pathway.63 In addition, platelets can activate endothelial cells in patients with SCD.77 More recently, endothelial cells have been shown to be activated by hemolysis. In particular, heme can be transported to endothelial cells by circulating microparticles, activating endothelial cells through TLR4-mediated signaling.53,57 The activation of endothelial cells is critical in sickle cell vaso-occlusion.78 In a hypoxia/reoxygenation-induced vaso-occlusion model, the anti-inflammatory reagent dexamethasone significantly inhibits leukocyte-endothelium interaction and prevents vaso-occlusion by reducing the expression of adhesion molecules on endothelial cells.78 Similar beneficial effects are observed when VCAM-1 or ICAM-1 is blocked with mAbs.78 In another vaso-occlusion model induced by TNF-α and surgical trauma, a pan-selectin antagonist, GMI-1070, has been found to reverse acute vascular occlusion.79
Molecules that promote inflammation in SCD
Proinflammatory cytokines play critical roles in the pathophysiology of SCD. SCD patients exhibit higher levels of several pro-inflammatory cytokines, including TNF-α, IL-6, IL-1β, GM-CSF, IL-3, endothelin-1, and prostaglandin E2.80-84 Proinflammatory cytokines activate endothelial cells and leukocytes through the NF-κB pathway.66 The activation of the NF-κB pathway results in increased expression of endothelial adhesion molecules, such as ICAM-1, VCAM-1, P-selectin, and E-selectin, promoting leukocyte adhesion and triggering the ensuing vaso-occlusive events.82 For example, TNF-α alone with surgical trauma is enough to trigger lethal acute vaso-occlusion in transgenic SCD mice.35
Chemokines are a family of small molecules that can induce chemotaxis.85 Chemokines contribute to SCD pathogenesis by acting on leukocytes.59 In NY1DD SCD mice, higher levels of CXCL9 and CXCL10 are observed in the lung.70 These chemokines recruit CXCR3-expressing leukocytes, promoting inflammation and causing pulmonary dysfunction. Neutralization of CXCR3 with a mAb significantly alleviates pulmonary dysfunction in these animals.70 CXCL1, a neutrophil chemoattractant, is also a key inflammatory mediator of acute vaso-occlusion in Berkeley SCD mice.59 CXCL1 is elevated during HTR-induced VOC. Administration of recombinant CXCL1 alone triggers lethal VOC in SCD mice, and blockade of CXCL1 receptor, CXCR2, inhibits VOC in this model.59 Importantly, elevated levels of IL-8 or CXCL8 are observed in SCD patients during crises and have been proposed to be a VOC predictor.86,87 IL-8 may play a role in SCD pathophysiology by promoting neutrophil adhesion to endothelial cells.88
The coagulation cascade also plays an important role in promoting vascular inflammation in SCD.89 Increased tissue factor (TF) expression, exposure of phosphatidylserine on sickle RBCs, circulating microparticles, and activated platelets together can lead to a well-documented hypercoagulable state and thrombotic complications.90,91 Although direct evidence that coagulation pathways contribute to major disease manifestation such as vaso-occlusion is still lacking, emerging evidence suggests that hypercoagulation and chronic inflammation in SCD are correlated. For example, a recent study shows that induction of endothelial TF expression depends on the NF-κB pathway in peripheral mononuclear cells.92 In addition, the histone deacetylase inhibitor trichostatin A and the trichostatin A analog suberoylanilide hydroxaminc acid (ie, drugs with multimodal activities), reduces both TF and VCAM-1 expression in SCD pulmonary vascular endothelial cells with or without a hypoxia/reoxygenation challenge.93 Hydroxyurea treatment is also found to reduce several markers of coagulation activation.94,95 Furthermore, inhibition of TF has been shown to attenuate inflammation-associated vascular injury, as measured by plasma levels of IL-6, serum amyloid P, and soluble VCAM-1.96 Inhibition of TF also reduces pulmonary expression of monocyte chemoattractant protein-1, keratinocyte chemoattractant, and myeloperoxidase in two models of SCD (BERK and Townes).96 Similarly, the inhibition of FXa and deficiency of thrombin receptor, protease activated receptor-2, in nonhematopoietic cells attenuate systemic plasma levels of IL-6.97 More recently, it has been reported that genetic deletion of circulating prothrombin alleviates multiorgan pathologies in SCD mice.98 In addition, thrombospondin-1 can stimulate microparticle release from RBCs. These microparticles trigger reactive oxygen species production by endothelial cells, promote leukocyte adhesion, and induce endothelial apoptosis in a phosphatidylserine-dependent manner, leading to acute vaso-occlusive events in SCD mice.99
HMGB1 has been recently identified as a key player in promoting inflammation in SCD. HMGB1 is a danger-associated molecular pattern molecule released from activated immune cells and necrotic cells, which can activate the TLR4 signaling pathway.100 The levels of HMGB1 are elevated and further increase during VOC in both SCD patients and transgenic SCD mice. HMGB1 accounts for the majority of sickle cell plasma-induced TLR4 activity both in vitro and in vivo.101
Inflammation, neuropathy, and pain
Most patients with SCD experience moderate to severe pain throughout their lifetime. The refractory nature of pain and the development of steady, persistent chronic pain in some patients suggest that neuropathy may contribute to pain in SCD. In a recent analysis of descriptors of pain reported by patients, pain in SCD turns out to be nociceptive and neuropathic, contrary to common expectations that SCD pain is only nociceptive.102 This finding has triggered investigations to ascertain whether an adjuvant analgesic treatment can improve pain management. Interestingly, trifluoperazine, a potent Ca2+/calmodulin protein kinase IIα inhibitor commonly used to treat neuropathic pain, shows significant beneficial effects on SCD patients, suggesting a role for neuropathy in the pathogenesis of SCD pain.103
Studies in SCD animal models have revealed neurochemical alterations in SCD. Similar to human patients, SCD mice exhibit altered sensitivity to pain as demonstrated by musculoskeletal and cutaneous hyperalgesia. Peripheral nerves and blood vessels are structurally altered in the skin, with decreased expression of υ-opioid receptors and increased calcitonin gene-related peptide and substance P immunoreactivity.104 Interestingly, activators of neuropathic and inflammatory pain, including p38, signal transducer and activator of transcription 3, and mitogen-activated protein kinase, exhibit increased phosphorylation, correlating with increases in COX-2, IL-6, and TLR4 levels in the spinal cord.104 Further studies identify a direct role for inflammation in pain pathophysiology in SCD, where mast cells promote neurogenic inflammation and nociceptor activation through the release of substance P in the skin and dorsal root ganglion. Targeting mast cells by genetic manipulation or pharmacologic inhibition effectively alleviates pain in SCD mice.105 These studies therefore suggest that neurogenic inflammation or neuropathy may be novel targets for pain management in SCD.
Emerging therapies targeting neutrophils, platelets, and inflammatory pathways in SCD
Since polymerization of the abnormal Hb represents a key step in the pathophysiology of SCD, it has been an important therapeutic target for over 5 decades. Any agents directly inhibiting this step, by HbF induction or by other mechanisms, will have numerous downstream effects on adhesion and inflammation. Our current understanding of the critical role of other cell types besides RBCs and key inflammatory processes, have led to the development of novel targeted therapies that are summarized here.
Targeting neutrophils
Hydroxyurea, the only FDA-approved therapy for SCD, is a potent inducer of HbF.106 However, hydroxyurea has multiple mechanisms by which it produces its clinical response. The Evidence-Based Management of Sickle Cell Disease: Expert Panel Report, 2014 (available at: http://www.nhlbi.nih.gov/health-pro/guidelines/sickle-cell-disease-guidelines/) recommends dose escalation of hydroxyurea to achieve mild myelosuppression (absolute neutrophil count, 2000 μL to 4000 μL) based on aggregate clinical and laboratory parameters. This is often accompanied by a reduction in baseline thrombocytosis. Although both of these effects may independently contribute to its clinical efficacy, they also affect the maximum tolerated dose, a clear predictor of HbF response.107 It is also interesting to note that short-term administration of either hydroxyurea or the phosphodiesterase 9 (PDE9) inhibitor ameliorates VOC in a SCD murine model via NO-mediated cGMP elevating effects that lead to reduced neutrophil adhesion and heterotypic RBC-neutrophil interactions. This suggests that hydroxyurea may have an immediate benefit in the setting of VOC.26 PDE9 is highly expressed in hematopoietic cells and is thus a promising therapeutic target. PF04447943, a PDE9 inhibitor, is currently being evaluated in a phase 1 study (#NCT02114203).
Rivipansel (GMI-1070), a synthetic pan-selectin inhibitor, has been shown to inhibit predominantly E-selectin–mediated leukocyte adhesion and dramatically reduce RBC-leukocyte interactions, leading to improved blood flow and prolonged survival in SCD mice during VOC.79 In a phase 1 clinical trial, GMI-1070 has been well tolerated without significant adverse effects. SCD patients receiving GMI-1070 exhibited a modest increase in total peripheral white blood cell count without clinical symptoms.108 Results of a prospective multicenter, randomized, placebo-controlled, double-blind, phase 2 study of 76 SCD patients with acute VOC reveals a positive, but nonsignificant trend, in the primary end point of time to VOC resolution in GMI1070 compared with placebo, and a clinically significant decline of 63 hours (48%) in median values.109 In addition, an 83% reduction of opioid use (P = .01) in the active arm has been noted. Currently, a phase 3 clinical trial of rivipansel in SCD is registered (#NCT02187003).
Intravenous immunoglobulin (IVIG) can reverse acute VOC in preclinical models by rapidly inhibiting neutrophil adhesion to the endothelium and abrogating RBC-neutrophil interactions.110,111 IVIG beneficial signaling is mediated by FcγRIII receptors, the only Fc receptor expressed on murine neutrophils, resulting in the recruitment of Src homology 2-containing tyrosine phosphatase-1 (SHP-1) and the inhibition of adhesion, and αMβ2 integrin activation. The protective effects of IVIG are abrogated in SHP-1–deficient mice, suggesting an important role of SHP-1 signaling in regulating neutrophil adhesion and activation.112 IVIG administration in SCD patients in a phase 1 study demonstrates no significant adverse events with doses up to 800 mg/kg, indicating safety and tolerability in acute VOC.113 Because lower doses show better biomarker and clinical efficacy profiles, the 400 mg/kg dose is selected for further study in an ongoing phase 2 trial (#NCT01757418).
Numerous preclinical and early phase clinical studies have demonstrated the benefit of targeting P-selectin.114 The efficacy of SelG1, a humanized anti–P-selectin mAb, in reduction of pain crises rates is currently being tested in an ongoing phase 2 trial (#NCT01895361). Heparins also act via the inhibition of P-selectin–mediated adhesion,115 in addition to inhibition of ligand binding to leukocyte integrin Mac-1116 and other anti-inflammatory effects.117 This is in addition to their expected anticoagulant effect. Tinzaparin, a low molecular weight heparin, has been studied in a randomized double-blind clinical trial. A total of 253 subjects were enrolled, aged 12 years and older, in a study in which reduced duration of VOC and no severe bleeding complications were reported.118 A feasibility study of unfractionated heparin in ACS is currently recruiting (#NCT02098993).
Targeting platelets
Prasugrel is a thienopyridine P2Y12 adenosine diphosphate (ADP) receptor antagonist that inhibits ADP-mediated platelet activation and aggregation. Randomized double-blind placebo-controlled phase 2 studies to examine safety have been completed in adults.119 Mean pain rates (percentage of days with pain) and intensity in the prasugrel arm are decreased compared with placebo. However, these reductions do not reach statistical significance. Platelet surface P-selectin and plasma soluble P-selectin, biomarkers of platelet and endothelial activation, are significantly reduced in SCD patients receiving prasugrel compared with placebo. Prasugrel is generally well tolerated and a phase 3 trial in children is ongoing (#NCT01794000). Ticagrelor is a novel antiplatelet agent that also inhibits ADP-mediated platelet activation, but unlike prasugrel, does not require metabolic activation. A phase 2 trial is currently registered (#NCT02482298).
Targeting inflammation
Regadenoson is an A2AR agonist reported in a phase 1 study in SCD patients to reduce iNKT cell activation to levels noted in healthy controls and SCD patients at baseline.75 Notably, iNKT cell activation is associated with increased phosphorylation of NF-κB p65, increased expression of A2AR, and higher levels of IFN-γ. Although NF-κB p65 phosphorylation was reduced to baseline levels, the reduction in A2AR expression and IFN-γ levels did not reach statistical significance.75 No toxicity has been noted. Based on these positive findings in the biological end points, a phase 2 randomized placebo-controlled trial is currently recruiting (#NCT01788631).
Several additional agents targeting inflammation via modulation of oxidant stress, NO bioavailability, coagulation, and inflammatory cytokines are listed in Table 1. Of note, a randomized placebo-controlled double-blind trial of ω-3 fatty acids conducted at a single center in Sudan shows promising results. Ω-3 treatment reduces VOC events and transfusions in enrolled patients (n = 140) monitored for a year.120 This well-tolerated therapy warrants further study. Glutamine enhances nicotinamide adenine dinucleotide in sickle RBCs.121 A recently completed phase 3 trial of glutamine in SCD (#NCT01179217) was conducted at 31 sites in the United States and enrolled 230 patients. A statistically significant reduction in pain crises and hospitalization is apparent in the treatment arm, with improvements in several secondary outcomes including the rates of ACS.
Conclusion
In summary, advances in the understanding of the pathophysiology of sickle VOC have led to several new exciting agents that are currently being evaluated. Given the complexity and multiplicity of events leading to the vaso-occlusive event, it is likely that a multitargeted multimodal approach will be required to achieve the best outcome.
Acknowledgments
This work was supported by a predoctoral fellowship from the American Heart Association (15PRE23010014) (D.Z.) and R01 grants from the National Institutes of Health, National Heart, Lung, and Blood Institute (HL069438 and HL116340) (P.S.F.) and National Institute of Diabetes and Digestive and Kidney Diseases (DK056638) (P.S.F.).
Authorship
Contribution: D.Z., C.X., D.M., and P.S.F. wrote the manuscript.
Conflict-of-interest disclosure: P.S.F. was a consultant and his laboratory has received funding from GlaxoSmithKline and NKT Therapeutics. The remaining authors declare no competing financial interests.
Correspondence: Paul S. Frenette, Albert Einstein College of Medicine, 1301 Morris Park Ave, Price Center, Room 101B, New York, NY 10461; e-mail: paul.frenette@einstein.yu.edu.
References
Author notes
D.Z., C.X., and D.M. contributed equally to this study.