In this issue of Blood, Lahoz-Beneytez et al use deuterium as a nonradioactive tracer to study neutrophil kinetics; their analysis shows that neutrophils originate from a large marrow progenitor pool and have a rapid blood turnover.1
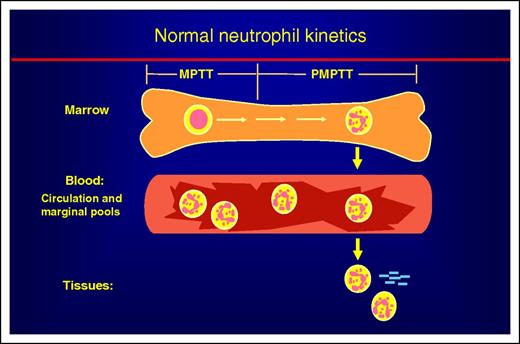
Schematic view of neutrophil kinetics. Neutrophil kinetics divides into 3 phases: marrow, blood, and tissues. In the marrow, developing neutrophils spend about half their time in the mitotic pool as myeloblasts, promyelocytes, myelocytes. The time to transit this pool is the mitotic pool transit time (MPPT). They then enter the postmitotic pool, becoming sequentially metamyelocytes, bands, and then neutrophils. The time for the neutrophil to pass through these stages is referred to as the postmitotic pool transit time (PMPTT). Neutrophils in the circulation are about equally divided between the circulating and marginal pools. Neutrophils leave the circulation and enter the tissues at inflammatory sites or become effete cells and are removed by macrophages in the spleen, marrow, and other tissues.
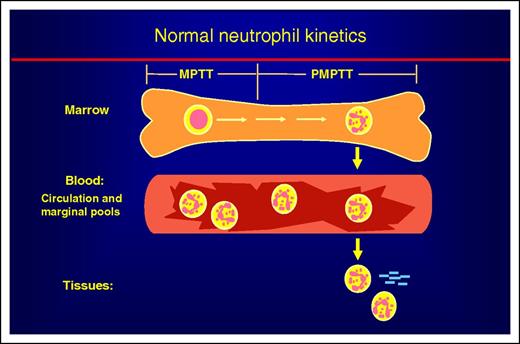
Schematic view of neutrophil kinetics. Neutrophil kinetics divides into 3 phases: marrow, blood, and tissues. In the marrow, developing neutrophils spend about half their time in the mitotic pool as myeloblasts, promyelocytes, myelocytes. The time to transit this pool is the mitotic pool transit time (MPPT). They then enter the postmitotic pool, becoming sequentially metamyelocytes, bands, and then neutrophils. The time for the neutrophil to pass through these stages is referred to as the postmitotic pool transit time (PMPTT). Neutrophils in the circulation are about equally divided between the circulating and marginal pools. Neutrophils leave the circulation and enter the tissues at inflammatory sites or become effete cells and are removed by macrophages in the spleen, marrow, and other tissues.
For more than 2 centuries, hematologists have tried to understand the dynamics of neutrophil production and the transit of these cells from the marrow through the blood to tissues. Progress in this field occurred only very gradually. In the 1840s, Addison described the formation of pus by white blood cells and Jones linked this phenomenon to leukocyte margination at a site of inflammation.2 Subsequently, Ehrlich provided the classic description of the morphology of stained blood cells and leukocyte changes with infections.3 For the next half century, most of the noteworthy reports in this field were descriptive, for example, conditions associated with neutropenia or neutrophilia, until the advent of radioisotopic studies in the “atomic age” of the 1950s.
As is well known, ionizing radiation and toxic chemicals, for example, external radiation and nitrogen mustard, have major effects on blood cell counts, rapidly causing neutropenia. It was primarily these observations that led researchers to conclude that neutrophils must have a very short life span. These same observations prompted hematologists to begin to study the use of radioactive isotopes, particularly phosphorus 32 (32P), as a tracer to study blood cell formation. In 1954, the Norwegian investigator Ottesen reported that the time required for 32P-labeled neutrophils to transit the postmitotic neutrophil compartment in the marrow and enter the blood was about 5 to 6 days.4 These studies were done by infusion of the isotope to label DNA of dividing cells and then serial separation of blood neutrophils after the infusion. This work also suggested that neutrophils rapidly leave the blood and disappear into the tissues. More sophisticated experiments in dogs and in humans soon followed, confirming these findings.5 Cartwright, Athens, and Wintrobe then led a decade of excellent work using the 32P-labeled diisopropyl fluorophosphate (DFP) to study neutrophil kinetics.6 They established values for pool sizes of neutrophils and their precursors from the marrow to the blood and tissues and also the concept and relative proportions of the circulating and marginal blood neutrophil pools. Subsequently, Dancey, Deubelbeiss, Harker, and Finch conducted the most comprehensive and quantitative studies of neutrophil kinetics ever performed in humans using 59Fe, 32P DFP, 3H thymidine and carefully performed bone marrow biopsies.7 This work established norms for neutrophil production and turnover and showed that at least the late phase of neutrophil production is very efficient; that is, cells entering the myelocyte-metamyelocyte stage will mature to become blood neutrophils. The methods established by these investigators were later used to show how myeloid growth factors increase neutrophil production, accelerate the release of neutrophils from the marrow to the blood, and prolonged the blood half-life of these cells.8
Over recent years, increasing concerns have arisen about the use of radioactive tracers in human investigations, particularly the use of even tracer amounts of 32P. Many of the radioisotopes previously used have become unavailable for clinical research. The search for alternatives with less potential hazard continues. 99Tc is used in nuclear medicine as a label for neutrophils to find sites of infection, but it is not suitable for research studies of neutrophil kinetics because the necessary separation and washing of the neutrophils is sufficiently damaging to the cells to disturb their natural transit in the blood. By contrast, 32P DFP (used in Craddock et al5 and Cartwright et al6 ) or 3H DFP (used in Price et al8 ) labels the serine proteases of neutrophils selectively, so that the labeling can be done with whole blood, protecting the cells from the damaging effects of washing. Neither of the isotopes is currently available to study neutrophil kinetics.
In 2010, Pillay et al reported studies using an infusion of deuterium labeled “heavy” water to study neutrophil kinetics.9 The methods for the clinical study were similar to those of previous investigators (Ottesen, Craddock, Athens, and Price). It was surprising that this new study reported a far longer blood half-life for neutrophils than any of the previous studies. Li et al raised questions about the assumptions made in analyzing the data and offered an alternative approach to analyzing the data which yielded results similar to those previously published.10 In this issue, Lahoz-Beneytez et al report a new study, using deuterium-labeled glucose and deuterium-labeled water. They measured the neutrophil emergence time and got identical results to those of Ottesen and the other investigators. Analyzing data using a 2-compartment model, they confirmed the old concepts of a large marrow pool of myeloid cells and a much smaller blood pool with rapid turnover.
Why is this study important? Understanding normal hematopoietic cell production is a foundation for understanding blood diseases (see figure). Proper diagnosis and treatment of neutropenia and neutrophilia rest on a physiological understanding of the normal process and what can go wrong with it. This new report by Lahoz-Beneytez et al “clears the air” from a previous report with good data but a flawed analysis. From another perspective, there is a common tendency to cite the latest report on a research topic, rather than an older study. As a “seasoned” investigator, it is satisfying to see that in this case the old studies, even those more than 50 years old, had it right.
Conflict-of-interest disclosure: The author declares no competing financial interests.