Key Points
With conventional immunosuppression, the incidence of chronic GVHD is higher after transplantation of mobilized blood compared with marrow.
Administration of cyclophosphamide after mobilized blood cell transplantation is associated with a low incidence of chronic GVHD.
Abstract
The cumulative incidence of National Institutes of Health (NIH)-defined chronic graft-versus-host disease (GVHD) requiring systemic treatment is ∼35% at 1 year after transplantation of granulocyte colony-stimulating factor (G-CSF)–mobilized blood cells from HLA-matched related or unrelated donors. We hypothesized that high-dose cyclophosphamide given after G-CSF–mobilized blood cell transplantation would reduce the cumulative 1-year incidence of chronic GVHD to 15% or less. Forty-three patients with high-risk hematologic malignancies (median age, 43 years) were enrolled between December 2011 and September 2013. Twelve (28%) received grafts from related donors, and 31 (72%) received grafts from unrelated donors. Pretransplant conditioning consisted of fludarabine and targeted busulfan (n = 25) or total body irradiation (≥12 Gy; n = 18). Cyclophosphamide was given at 50 mg/kg per day on days 3 and 4 after transplantation, followed by cyclosporine starting on day 5. The cumulative 1-year incidence of NIH-defined chronic GVHD was 16% (95% confidence interval, 5-28%). The cumulative incidence estimates of grades 2-4 and 3-4 acute GVHD were 77% and 0%, respectively. At 2 years, the cumulative incidence estimates of nonrelapse mortality and recurrent malignancy were 14% and 17%, respectively, and overall survival was projected at 70%. Of the 42 patients followed for ≥1 year, 21 (50%) were relapse-free and alive without systemic immunosuppression at 1 year after transplantation. Thus, myeloablative pretransplant conditioning can be safely combined with high-dose cyclophosphamide after transplantation, and the risk of chronic GVHD associated with HLA-matched mobilized blood cell grafts can be substantially reduced. This trial was registered at www.clinicaltrials.gov as #NCT01427881.
Introduction
Graft-versus-host disease (GVHD) continues to compromise the overall success of allogeneic hematopoietic cell transplantation (HCT). Although rates and severity of acute GVHD have decreased with improvements in donor selection criteria, pharmacologic prophylaxes, and supportive care, rates of chronic GVHD have remained remarkably stable at 35% to 50% for many years.1 The median duration of systemic immunosuppressive treatment of chronic GVHD is ∼2.5 years after bone marrow transplantation (BMT) and 3.5 years after mobilized blood cell transplantation. Because chronic GVHD contributes to long-term morbidity and mortality after allo-HCT, strategies that prevent this complication without compromising beneficial graft-versus-tumor (GVT) effects are urgently needed.
Recent studies have convincingly shown that high-dose cyclophosphamide (Cy) given early after HCT does not compromise engraftment and can decrease the risk of chronic GVHD. In studies of HLA-haploidentical BMT with reduced-intensity conditioning, Cy was given at 50 mg/kg on days 3 and 4 or only on day 3 followed by tacrolimus and mycophenolate mofetil (MMF).2-4 The cumulative incidence frequencies of grades II-IV GVHD and grades III-IV GVHD were 34% and 6%; the cumulative incidence frequencies of chronic GVHD were 25% after a single dose of Cy and only 5% after 2 doses of Cy.3
In a prospective phase 2 study, high-dose Cy was given as single-agent prophylaxis on days 3 and 4 after HLA-matched related or unrelated BMT with busulfan/cyclophosphamide conditioning.5 The cumulative incidence frequencies of grades II-IV GVHD, grades III-IV GVHD, and chronic GVHD were 43%, 10%, and 10%, respectively. In a follow-up study, high-dose Cy was given on days 3 and 4 after HLA-matched related or unrelated BMT with busulfan/fludarabine conditioning. The cumulative incidence frequencies of grades II-IV GVHD, grades III-IV GVHD, and chronic GVHD were 51%, 15%, and 14%, respectively. Taken together, these results show that the cumulative incidence of chronic GVHD is consistently <15% when high-dose Cy is given as the sole GVHD prophylaxis after HLA-matched related or unrelated BMT.6
Compared with bone marrow, the relative ease of procurement combined with faster neutrophil and platelet reconstitution after transplant have made growth factor–mobilized blood a popular graft source for allogeneic HCT. When a calcineurin inhibitor and an antimetabolite are used for immunosuppression after allogeneic HCT, however, the advantage of earlier hematopoietic reconstitution is offset by a higher risk and longer duration of chronic GVHD.7-9 The present study was therefore designed to address this problem by determining whether the low incidence of chronic GVHD associated with the use of high-dose Cy after BMT could be replicated in patients who received mobilized blood cell grafts. Cyclosporine (CSP) was added in an effort to reduce the risk of grades 3-4 acute GVHD that had been reported to range from 10% to 15% in earlier studies of HLA-matched BMT where high-dose Cy was the sole means of postgrafting immunosuppression.5,6
Methods
Study design
The primary end point of the study was the cumulative incidence of chronic GVHD (defined by National Institutes of Health [NIH] criteria10 ) requiring systemic immunosuppressive treatment compared with the historic rate of 35%.11 Major secondary end points included grades II-IV and III-IV acute GVHD, need for systemic treatment of acute GVHD, persistent or recurrent malignancy after HCT, and disease-free and overall survival.
Patients
Eligibility criteria included the following: patients 65 years of age or younger with a high-risk hematologic malignancy as defined (supplemental Data available on the Blood Web site); peripheral blood stem cell donor who was either a genotypically HLA-identical sibling, a phenotypically HLA-matched first-degree relative, or an unrelated donor who was matched with the patient at HLA-A, B, C, and DRB1 by molecular methods. Initially, unrelated donors mismatched with the patient at a single class I antigen were eligible for the study. After the first transplant from an HLA-A-antigen–mismatched unrelated donor resulted in poor graft function requiring a second transplant, the protocol was amended to exclude HLA-mismatched donors. Exclusion criteria included the following: patients who had received prior autologous or allogeneic HCT, an Eastern Cooperative Oncology Group performance status >2, uncontrolled infections, pregnancy, or organ dysfunction as defined (supplemental Data). The institutional review board of the Fred Hutchinson Cancer Research Center approved this study, and all patients provided written informed consent before enrollment, in accordance with the Declaration of Helsinki. The characteristics of patients, donors, and transplant regimens are summarized in Table 1.
Preparative and immunosuppressive regimens
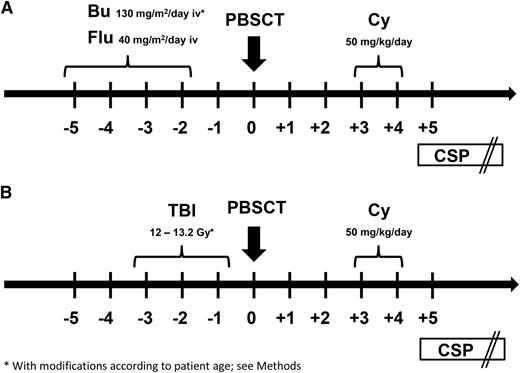
Participants received 1 of 2 preparative regimens: (1) FLU/TBU, which consisted of fludarabine 40 mg/m2 per day intravenously followed by targeted intravenous busulfan on days −5 until −2 or (2) fractionated total body irradiation (TBI) at a cumulative dose of 12 Gy or higher (12 Gy, n = 13; 13.2 Gy, n = 5) on days −5 to −2 (Figure 1). In general, patients with myeloid malignancies received the FLU/TBU regimen, whereas those with lymphoid malignancies received TBI. The initial intravenous busulfan dose was 130 mg/m2 per day for patients 18 years of age or older; 0.8 mg/kg every 6 hours for patients from 10 to 17 years of age; and 1 mg/kg every 6 hours for patients <10 years of age) (n = 25). All subsequent busulfan doses were adjusted in real time to targeted steady-state concentrations of 800 to 900 ng/mL.12 Granulocyte colony-stimulating factor (G-CSF)–mobilized peripheral blood mononuclear cells were collected by leukapheresis on days −1 and 0, and the products from both days were infused on day 0. A second collection on day 0 was not required if the collection on day –1 yielded ≥5 × 106 CD34 cells/kg recipient weight. Cy was given at 50 mg/kg per day intravenously on days 3 and 4 after graft infusion. Mesna was administered on days 3 and 4 for urothelial protection. An intravenous loading dose of CSP was given on day 5, followed by subsequent twice daily dosing adjusted to maintain whole blood trough concentrations at 120 to 360 ng/mL. In the absence of GVHD, CSP doses were tapered from day 56 through day 126.
Preparative regimens. Participants in this study (n = 43) received 1 of 2 preparative regimens: (A) FLU/TBU (n = 25), which consisted of intravenous fludarabine 40 mg/m2 per day in combination with intravenous busulfan on days −5 until −2 (busulfan was targeted to steady-state concentrations of 800 to 900 ng/mL) or (B) fractionated TBI (n = 18) at a cumulative dose of 12 Gy or higher (12 Gy, n = 13; 13.2 Gy, n = 5) on days −5 until −2. In general, patients with myeloid malignancies received the FLU/TBU regimen, whereas those with lymphoid malignancies received TBI. G-CSF–mobilized peripheral blood mononuclear cells (≥5 × 106 CD34 cells/kg recipient weight) were infused on day 0. Cyclophosphamide (Cy) was given at 50 mg/kg per day intravenously on days 3 and 4 after transplant. An intravenous loading dose of CSP was given on day 5, followed by subsequent twice daily dosing adjusted to maintain whole blood trough concentrations at 120 to 360 ng/mL. In the absence of GVHD, CSP doses were tapered from day 56 through day 126.
Preparative regimens. Participants in this study (n = 43) received 1 of 2 preparative regimens: (A) FLU/TBU (n = 25), which consisted of intravenous fludarabine 40 mg/m2 per day in combination with intravenous busulfan on days −5 until −2 (busulfan was targeted to steady-state concentrations of 800 to 900 ng/mL) or (B) fractionated TBI (n = 18) at a cumulative dose of 12 Gy or higher (12 Gy, n = 13; 13.2 Gy, n = 5) on days −5 until −2. In general, patients with myeloid malignancies received the FLU/TBU regimen, whereas those with lymphoid malignancies received TBI. G-CSF–mobilized peripheral blood mononuclear cells (≥5 × 106 CD34 cells/kg recipient weight) were infused on day 0. Cyclophosphamide (Cy) was given at 50 mg/kg per day intravenously on days 3 and 4 after transplant. An intravenous loading dose of CSP was given on day 5, followed by subsequent twice daily dosing adjusted to maintain whole blood trough concentrations at 120 to 360 ng/mL. In the absence of GVHD, CSP doses were tapered from day 56 through day 126.
Cy pharmacokinetics
Cy pharmacokinetics were evaluated with each Cy dose in 31 patients. For logistical reasons, 12 patients did not contribute samples to this analysis. Samples were collected at 1 (end of infusion), 2, 4, 8, 16, and 24 hours from the start of the infusion. The 24-hour samples were collected before the start of the subsequent dose. Samples were placed at 4°C within 5 minutes of collection and were processed within 18 hours. Concentrations of Cy, carboxyethylphosphoramide mustard (CEPM), deschloroethylcyclophosphamide (DCCy), and ketocyclophosphamide (KetoCY)were measured as previously reported,13 except using liquid chromatography with tandem mass spectrometry. The dynamic range of the assays of Cy and its metabolites were as follows: Cy, 0.96 to 192 µM; CEPM, 0.043 to 17 µM; DCCy, 0.063 to 25 µM; KetoCY, 0.022 to 9.1 µM. All assays had an interday precision within 10%. The plasma exposure is the area under the plasma concentration – time curve from time 0 to 48 hours (AUC0-48h). The AUCs were estimated according to noncompartmental modeling with Phoenix (Pharsight, St. Louis, MO).
Treatment of GVHD
First-line treatment of acute GVHD was prednisone 0.5 to 2 mg/kg per day, according to severity of symptoms.14 In patients with gastrointestinal GVHD, systemic prednisone treatment was combined with oral beclomethasone dipropionate emulsion (4 mg/day) and enteric-coated budesonide (6 mg/day). Treatment with CSP was continued as tolerated. Prednisone doses were tapered as GVHD symptoms resolved. Decisions regarding the timing and choice of secondary therapy were made at the discretion of the attending physician.
Statistical analysis
Primary end point.
The primary end point of this study was the cumulative incidence of chronic GVHD at 1 year after transplantation. Chronic GVHD was defined by NIH criteria as requiring systemic immunosuppressive treatment. The cumulative incidence of this end point in patients prepared with myeloablative regimens after HCT from HLA-matched related or unrelated donors has been ∼35%.11 A reduction in the cumulative incidence of chronic GVHD from ∼35% to ∼15% at 1 year was considered a reasonable goal after HCT with growth factor–mobilized blood cells. A sample size of 42 patients provided 90% power to observe such a difference with a 1-sided 5% type 1 error. The analysis plan specified that survival rates at 1 year would be evaluated to determine whether a low cumulative incidence of chronic GVHD could be explained by an excessive number of early deaths.
Secondary end points.
Grades II-IV and II-IV acute GVHD, nonrelapse mortality (NRM), and recurrent or progressive malignancy were assessed with the use of cumulative incidence estimates. Disease-free and overall survival rates were evaluated as Kaplan-Meier estimates. Donor engraftment was assessed with descriptive statistics summarizing the median proportions of donor myeloid cells and T cells in the blood at day 28. In addition, the proportions of patients with full and mixed chimerism were summarized. Hematologic recovery was assessed through descriptive statistics summarizing the median days to neutrophil and platelet recovery. Graft failure was assessed through descriptive statistics summarizing the incidence of primary graft failure and secondary graft failure.
Results
Patient, donor, and allograft characteristics
Patient, donor and allograft characteristics are summarized in Table 1. A total of 43 patients with high-risk hematologic malignancies were treated between December 2011 and September 2013 at the Fred Hutchinson Cancer Research Center. The median follow-up for surviving patients is 23 (range, 6-38) months. The median patient age was 43 (range, 3-66) years. Twenty-two patients (51%) had disease in complete remission without minimal residual disease and 21 (49%) had evidence of minimal residual disease (MRD) by virtue of flow cytometry, cytogenetic analysis or fluorescence in situ hybridization, or disease not in morphologic remission at the time of transplant. Twenty-five patients (58%) received fludarabine/TBU and 18 patients received TBI (≥12 Gy). Twelve patients (28%) received allografts from HLA-identical siblings, 30 (70%) from 10/10 HLA-matched unrelated donors, and 1 (2%) from an HLA A-antigen–mismatched unrelated donor.
Engraftment
The median times to neutrophil engraftment to 0.5 × 103/μL and platelet engraftment to 20 × 103/μL were 19 (range, 16-37) days and 14 (range, 10-47) days, respectively, for patients with primary engraftment. One patient had primary engraftment failure. This patient had familial myelodysplastic syndrome (MDS; GATA2 mutation) and had received a graft from an HLA A-antigen–mismatched unrelated donor. Peripheral blood chimerism for both CD3 and CD33 consistently showed >95% donor signal. The patient received a second transplant using 2 umbilical cord blood units after reduced intensity conditioning. Engraftment did not occur, and the patient died with multiorgan failure on day 41 after the second transplant. Even though causality between the transplant regimen and engraftment failure in this patient was never established, the protocol was subsequently modified to exclude patients with HLA-antigen–mismatched donors.
GVHD
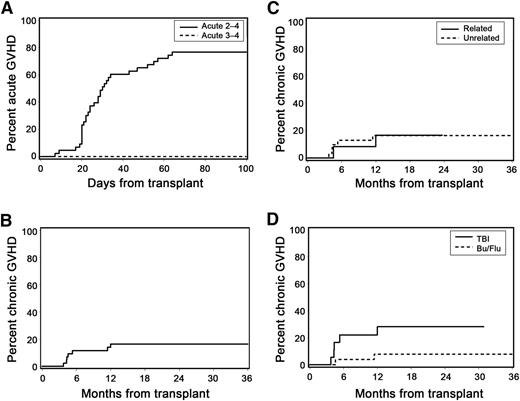
The cumulative incidence frequencies of grades 2-4 and 3-4 acute GVHD at day 100 were 77% and 0%, respectively (Figure 2A). Among patients with acute GVHD, 19% developed grade 2b symptoms characterized by rash involving >50% of the body surface. No patient with acute GVHD developed stage 2 gastrointestinal manifestations (stool volume ≥1.0 L/day). Acute GVHD was treated with glucocorticoids in all patients (initial methylprednisolone-equivalent dose, 0.5–2.0 mg/kg per day). At 1 year after transplant, only 3 patients continued immunosuppressive treatment for management of acute GVHD without meeting criteria for NIH-defined chronic GVHD. There was no identifiable association between patient age and risk of acute GVHD (data not shown).
Acute and chronic GVHD. (A) Cumulative incidence of acute GVHD. (B) Cumulative incidence of NIH-defined chronic GVHD (at 1 year: 16%; 95% CI, 5-28%). (C) Cumulative incidence of NIH-defined chronic GVHD according to donor type. (D) Cumulative incidence of NIH-defined chronic GVHD according to preparative regimen (HR, 0.55; 95% CI, 0.3-1.1; P = .09).
Acute and chronic GVHD. (A) Cumulative incidence of acute GVHD. (B) Cumulative incidence of NIH-defined chronic GVHD (at 1 year: 16%; 95% CI, 5-28%). (C) Cumulative incidence of NIH-defined chronic GVHD according to donor type. (D) Cumulative incidence of NIH-defined chronic GVHD according to preparative regimen (HR, 0.55; 95% CI, 0.3-1.1; P = .09).
The cumulative incidence of NIH-defined chronic GVHD requiring systemic immunosuppressive treatment at 1 year, which was the primary end point of the study, was 16% (95% confidence interval [CI], 5-28%; Figure 2B). All cases of chronic GVHD occurred within the first year after transplant. We also analyzed the cumulative incidence of extensive chronic GVHD defined by traditional criteria,15 although this outcome was not a formal end point of this study. At 1 year, the cumulative incidence of extensive chronic GVHD was 30% (95% CI, 17-44%). The following variables were evaluated as possible predictors of chronic GVHD but were not statistically significant: unrelated donor vs related donor (hazard ratio [HR], 1.11; 95% CI, 0.2-5.7; P = .90; Figure 2C); FLU/TBU vs TBI (HR, 0.27; 95% CI, 0.1-1.4; P = .12; Figure 2D); and CD34 cell dose >median vs <median of 8.25 × 106/kg (HR, 1.38; 95% CI, 0.3-6.2; P = .67). Due to the low event rate, an analysis to assess the possible impact of patient age on chronic GVHD was not performed.
At 12 months after transplant, 10 patients (23%) had died, and 33 (77%) were alive. Five patients died with relapse and 5 had NRM. At the time of death, 6 of the 10 patients were receiving systemic immunosuppressive medications either for GVHD treatment (n = 4) or for GVHD prophylaxis (n = 2). Twenty-three of the 33 patients (70%) alive at 12 months had discontinued all systemic immunosuppression, and 10 (30%) were receiving systemic immunosuppressive medications either for acute (n = 3) or chronic (n = 7) GVHD. With a median follow-up of 13.5 (range, 6.7-28.5) months, all 7 patients who had NIH-defined chronic GVHD were alive, and 6 were still receiving systemic immunosuppressive therapy.
Relapse, NRM, progression-free survival, and survival
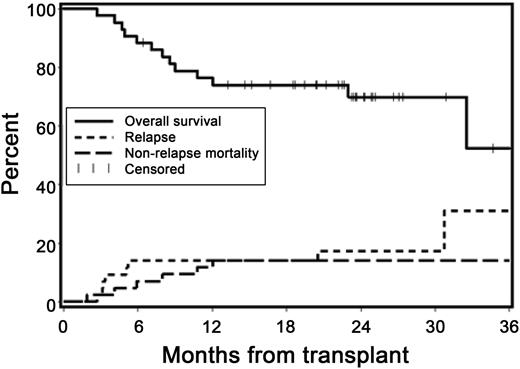
At 2 years, the cumulative incidences of recurrent malignancy and NRM were 17% (95% CI, 5-29%) and 14% (95% CI, 4-25%), respectively (Figure 3). The Kaplan-Meier overall survival estimate at 2 years was 70% (95% CI, 55-85%), and the estimate of progression-free survival at 2 years was 69% (95% CI, 54-83%). Of the 42 patients (98%) followed for ≥1 year, 21 (50%) were relapse-free and alive without systemic immunosuppression at 1 year after HCT.
Overall survival, relapse, and NRM. Kaplan-Meier estimates of overall survival and cumulative incidence curves of NRM and relapse. At 2 years, the cumulative incidence estimates of NRM and recurrent malignancy were 14% and 17%, respectively, and survival was projected at 70%.
Overall survival, relapse, and NRM. Kaplan-Meier estimates of overall survival and cumulative incidence curves of NRM and relapse. At 2 years, the cumulative incidence estimates of NRM and recurrent malignancy were 14% and 17%, respectively, and survival was projected at 70%.
Exploratory analysis showed that patients prepared with high-dose TBI had a significantly higher 2-year survival likelihood than those prepared with FLU/TBU (83% vs 59%; supplemental Figure 1). The interpretation of these results is confounded by the preponderance of high-risk myeloid malignancies and the older age of patients treated with FLU/TBU (median age, 52 vs 23.5 years; P = .05). Patients with leukemia or MDS who had MRD at the time of transplant (n = 14) had significantly worse progression-free survival (38% vs 90%, P = .002) and overall survival (45% vs 85%, P = .009) compared with those who did not have MRD (n = 22; Figure 4).
Kaplan-Meier survival estimates according to remission status at the time of transplant. Minimal residual disease (MRD)-neg complete remission (CR): 22 patients (51%) had disease in complete remission without minimal residual disease (AML, n = 12; ALL, n = 8; MDS, n = 2). MRD-pos CR: 14 patients (33%) were in morphologic remission but had evidence of minimal residual disease by virtue of flow cytometry, cytogenetic analysis, or fluorescence in situ hybridization (AML, n = 5; ALL, n = 5; MDS, n = 3; chronic myeloid leukemia [CML], n = 1). Other: 7 patients (16%) had >5% marrow blasts or detectable lymphoma (MDS, n = 4; CML, n = 2; non-Hodgkin lymphoma, n = 1). (A) Progression-free survival. (B) Overall survival. Comparing patients with MRD-positive CR to those with MRD-negative CR, progression-free survival and overall survival were statistically significantly different (P = .002 and P = .009, respectively).
Kaplan-Meier survival estimates according to remission status at the time of transplant. Minimal residual disease (MRD)-neg complete remission (CR): 22 patients (51%) had disease in complete remission without minimal residual disease (AML, n = 12; ALL, n = 8; MDS, n = 2). MRD-pos CR: 14 patients (33%) were in morphologic remission but had evidence of minimal residual disease by virtue of flow cytometry, cytogenetic analysis, or fluorescence in situ hybridization (AML, n = 5; ALL, n = 5; MDS, n = 3; chronic myeloid leukemia [CML], n = 1). Other: 7 patients (16%) had >5% marrow blasts or detectable lymphoma (MDS, n = 4; CML, n = 2; non-Hodgkin lymphoma, n = 1). (A) Progression-free survival. (B) Overall survival. Comparing patients with MRD-positive CR to those with MRD-negative CR, progression-free survival and overall survival were statistically significantly different (P = .002 and P = .009, respectively).
Cy pharmacokinetics according to preparative regimen
Substantial variability in the pharmacokinetics of Cy and its metabolites was observed. The median and range of maximum plasma concentrations (Cmax) were 244 (196-410) μM for Cy, 17.6 (4.1-35.6) μM for CEPM, 9.9 (2.6-21.3) μM for DCCY, and 6.7 (1.9-15.9) μM for KetoCY. The median (range) of the AUC0-48h was 1156 (533-2722) μM⋅h for CY, 159 (64.1-248) μM⋅h for CEPM, 137 (47.6-336) μM⋅h for DCCY, and 93.3 (33.1-198) μM⋅h for KetoCY. According to the preparative regimen, the AUC0-48h of Cy was higher in patients treated with TBI without phenytoin (n = 9) than in those treated with FLU/TBU (n = 22; P < .0001) with phenytoin, whereas the AUCs of KetoCY were lower (P = .03; Figure 5). The AUCs of CEPM and DCCY did not show statistically significant differences according to the preparative regimen (P = .05 and 0.14; Figure 5). Because of the low event rate of chronic GVHD, a meaningful pharmacodynamic analysis of the impact of AUC on outcome was not possible.
Inductive effects of phenytoin (FLU/TBU; n = 22) compared with no phenytoin (TBI; n = 9) on cyclophosphamide metabolism. Boxplot shows median and interquartile ranges (25th and 75th percentile) of respective analyte AUC0-48h (μM⋅h). A statistically significant difference was observed between preparative regimens for the AUC of CY (P < .0001) and ketoCY (P = .03), but not the AUCs of CEPM and DCCY (P = .05 and 0.14; respectively).
Inductive effects of phenytoin (FLU/TBU; n = 22) compared with no phenytoin (TBI; n = 9) on cyclophosphamide metabolism. Boxplot shows median and interquartile ranges (25th and 75th percentile) of respective analyte AUC0-48h (μM⋅h). A statistically significant difference was observed between preparative regimens for the AUC of CY (P < .0001) and ketoCY (P = .03), but not the AUCs of CEPM and DCCY (P = .05 and 0.14; respectively).
Discussion
In this study, we explored the safety and efficacy of Cy-based GVHD-prophylaxis after HLA-matched related or unrelated mobilized blood cell transplantation with either FLU/TBU or TBI (≥12 Gy) myeloablative conditioning. The 16% cumulative incidence of NIH-defined chronic GVHD at 1 year was substantially lower than that observed in studies of HCT with either marrow or mobilized blood cells from HLA-matched related or unrelated donors. The incidence of grade 2 acute GVHD was high, but unlike previous studies with the use of high-dose Cy after HLA-matched BMT, we observed no cases of grades 3 or 4 acute GVHD and no cases of grade 2 acute GVHD with high-volume diarrhea (stool volume ≥1.0 L/day). The results showed low risks of NRM and recurrent malignancy, translating to an overall projected survival rate of 70% at 2 years. Of the patients followed for ≥1 year, 50% were relapse-free and alive without systemic immunosuppression at 1 year after HCT.
The use of mobilized blood cells has demonstrated benefits of easier procurement, more rapid engraftment, and superior control of high-risk malignancies. These benefits have made growth factor–mobilized blood the most common source of stem cells used at many transplant centers. The main disadvantage of using mobilized blood cell grafts, however, is the significant increase in risk and duration of chronic GVHD.7-9 We observed a low incidence of chronic GVHD in the current study, even though the proportion of the patients with HLA-matched related donors (30%) was lower than in the study used to set the benchmark (44%).11 Therefore, the results suggest that the risk of chronic GVHD associated with mobilized blood cell grafts can be substantially reduced by using high-dose Cy and CSP after HCT. By applying NIH criteria for comparing the incidence of chronic GVHD, the 16% incidence in our study (primary end point) is comparable to the 18% incidence when high-dose Cy was given after HLA-haploidentical transplantation of mobilized blood cells16 and the 10% to 14% incidence when high-dose Cy was given after HLA matched related or unrelated BMT.5,6 Although not a formal end point in this study, a statistically significant reduction in the risk of chronic GVHD to 30% (95% CI, 17-44%) was also observed after applying the traditional chronic GVHD grading system15 to our study population. In a contemporaneous control cohort given mobilized blood cell grafts from HLA-matched related or unrelated donors after myeloablative conditioning (n = 168), the cumulative incidence of chronic GVHD requiring systemic treatment at 1 year after HCT was 54% (M.M., unpublished data, 2015).
In previous studies testing high-dose Cy alone for immunosuppressive prophylaxis after HLA-matched related or unrelated BMT, the cumulative incidence rates of severe (grades 3-4) acute GVHD were between 10% and 15%.5,6 For this reason, our study regimen included immunosuppression with CSP initiated on posttransplant day 5, the day after the second dose of Cy. Although 77% of patients developed grade 2 acute GVHD in our study, we did not observe severe (grade 3-4) manifestations, and no patient developed stage 2 gastrointestinal involvement (stool volume ≥1 L/day). In addition, previous studies have shown that after HCT with myeloablative conditioning, grade 2 acute GVHD had no effect on survival but was associated with a decreased risk of relapse.17 The generally increased incidence of grade 2 acute GVHD reported at our center has been attributed to high diagnostic sensitivity and increased awareness that gut GVHD can occur without skin involvement.18 Thus, our findings suggest that the addition of a calcineurin inhibitor to high-dose Cy after HLA-matched growth factor–mobilized blood transplantation can significantly reduce the risk of severe acute GVHD. Nevertheless, optimizing the GVHD prophylactic regimen to reduce the risk of even mild-to-moderate acute GVHD would be desirable to further reduce the morbidity associated with this complication.
Approximately half of the patients had MRD or active disease at the time of HCT. Although absence of MRD was associated with superior progression-free survival and overall survival (Figure 4), our results suggest that effective prevention of chronic GVHD was not offset by an increased risk of recurrent malignancy. We speculate that the high incidence of mild grade 2 acute GVHD and low incidence of chronic GVHD reflect residual immunologic activity of donor cells against recipient cells that is sufficient to mediate GVL effects after myeloablative conditioning. If these findings can be confirmed in future studies, the practice of allo-HCT with mobilized blood cells could change with the adoption of high-dose Cy combined with a calcineurin inhibitor as the mainstay of posttransplant immunosuppressive prophylaxis.
In addition to FLU/TBU, high-dose TBI (≥12 Gy) was safely combined with high-dose posttransplant Cy in this study. The use of TBI allowed treatment of patients with diseases for which the FLU/TBU regimen would not be optimal, such as acute lymphoblastic leukemia (ALL), high-risk acute myeloid leukemia (AML), and lymphoma. Although 89% of patients given high-dose TBI/Cy received grafts from HLA-matched unrelated donors, the toxicity observed with this approach was exceptionally low (2-year NRM, <10%), resulting in a 2-year overall survival estimate of 90%. Given the promising results with high-dose TBI (≥12 Gy) and high-dose posttransplant Cy in this subset of study patients, larger confirmatory studies with this regimen are warranted.
Cy is a prodrug with complex metabolism. Dosing Cy by body weight led to considerable interpatient variability in the AUCs of Cy and its metabolites, consistent with our previous data.19 Formation of 4-hydroxycyclophosphamide (HCy) is critical, because this intermediate is transported intracellularly and subsequently metabolized to acrolein and phosphoramide mustard, the latter functioning as a bifunctional alkylator of DNA and the ultimate cytotoxic metabolite of Cy. HCy is detoxified to KetoCY by cytochrome P-450 and CEPM by aldehyde dehydrogenase 1.20,21 Notably, acrolein is the only Cy metabolite that inhibits aldehyde dehydrogenase 1 activity in vivo.20 Cy plasma exposure was lower in patients given FLU/TBU with phenytoin compared with those given TBI-based conditioning without phenytoin (Figure 5), but the heterogeneity of the patient population and the low event rates of chronic GVHD and relapse made it difficult to determine whether plasma exposure of Cy and its metabolites was associated with clinical outcomes. The lower exposure in patients given FLU/TBU is consistent with our prior data with TBU/Cy22 and presumably reflects the inductive effects of phenytoin, which was used to prevent busulfan-induced seizures. Although 3 to 4 days elapsed between the last phenytoin dose and the first posttransplant Cy dose, the inductive effects of phenytoin were unequivocal (Figure 5). Larger pharmacokinetic/dynamic studies might help elucidate the clinical relevance of variability of Cy metabolism.
In conclusion, high-dose Cy in combination with CSP after myeloablative HLA-matched mobilized blood cell transplantation resulted in low rates of both chronic GVHD and severe acute GVHD. In conjunction with high-dose pretransplant conditioning, the immunosuppressive regimen was safe, and effective GVHD-protection did not compromise control of underlying malignancy. If these findings are confirmed in future studies, HLA-matched mobilized blood cell transplantation may gain even greater acceptance and further replace marrow as a source of stem cells for most indications.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Helen Crawford for assistance with manuscript preparation and Dr Stephanie Lee with assistance in chronic GVHD data collection and interpretation. The authors also thank the physicians, nurses, physician assistants, nurse practitioners, pharmacists, and support staff caring for our patients and the patients who participated in this study.
This work was supported by National Institutes of Health, National Heart, Lung and Blood Institute grant HL108307, and National Cancer Institute grants CA078902, CA018029, CA015704, CA182963, and CA162059.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or its subsidiary Institutes and Centers.
Authorship
Contribution: M.M. designed the study, analyzed and interpreted data, and wrote the manuscript; T.F. assisted with data collection and edited the manuscript; P.V.O. assisted with study design and data interpretation and edited the manuscript; B.E.S. performed statistical analysis; J.S.M. performed the pharmacokinetic analysis, assisted with data interpretation, and edited the manuscript; P.A.C. assisted with data interpretation and edited the manuscript; M.E.D.F. assisted with data interpretation and edited the manuscript; R.S. assisted with data interpretation and edited the manuscript; F.R.A. assisted with data interpretation and edited the manuscript; and P.J.M. assisted with study design and data interpretation and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Marco Mielcarek, Fred Hutchinson Cancer Research Center, 1100 Fairview Ave N, Mail Stop D1-100, PO Box 19024, Seattle, WA 98109; e-mail: mmielcar@fhcrc.org.

![Figure 4. Kaplan-Meier survival estimates according to remission status at the time of transplant. Minimal residual disease (MRD)-neg complete remission (CR): 22 patients (51%) had disease in complete remission without minimal residual disease (AML, n = 12; ALL, n = 8; MDS, n = 2). MRD-pos CR: 14 patients (33%) were in morphologic remission but had evidence of minimal residual disease by virtue of flow cytometry, cytogenetic analysis, or fluorescence in situ hybridization (AML, n = 5; ALL, n = 5; MDS, n = 3; chronic myeloid leukemia [CML], n = 1). Other: 7 patients (16%) had >5% marrow blasts or detectable lymphoma (MDS, n = 4; CML, n = 2; non-Hodgkin lymphoma, n = 1). (A) Progression-free survival. (B) Overall survival. Comparing patients with MRD-positive CR to those with MRD-negative CR, progression-free survival and overall survival were statistically significantly different (P = .002 and P = .009, respectively).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/127/11/10.1182_blood-2015-10-672071/4/m_1502f4.jpeg?Expires=1769224552&Signature=cdCLEzHOAeEB0i8MFQwVZW8iN8z23yTLcxAl~ZiOIQpSntlqWf1J85m1115toZRzPz1UtUMObacEfNnzgJj~nR~FEYxFC8w2dzaBDRs8c9rOHqyPJT9vlrUun4a8pbsvDFeoGZfYH3zEB8b52DG0BIfIUj30idHsUoqgLqcR2XnpRiJphYMzYQfFrHZXL1AObOlHUhKZsk8hkOZuzUvKq7fNSdv8zvtBcAJkN~QSGDbliKV6zTlt~y80ZJqsRvbhBPrmhnp~M7r6teOFKwwW6~Ng8-7DDts35g2LDsWQw4xskjoY8H~Aq5kfqE0whuOQMK-4xWw0rHxBdb0SfvDnFA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

