Key Points
Coxiella burnetii is associated with an increased risk of lymphoma; its presence in the tumor microenvironment may favor lymphomagenesis.
Lymphoma has to be considered in patients with Q fever and lymphoid disorders, especially those with persistent focalized infections.
Abstract
Bacteria can induce human lymphomas, whereas lymphoproliferative disorders have been described in patients with Q fever. We observed a lymphoma in a patient with Q fever that prompted us to investigate the association between the 2 diseases. We screened 1468 consecutive patients of the 2004 to 2014 French National Referral Center for Q fever database. The standardized incidence ratios (SIRs) of diffuse large B-cell lymphoma (DLBCL) and follicular lymphoma (FL) were calculated comparatively to the 2012 Francim Registry. The presence of Coxiella burnetii was tested using immunofluorescence and fluorescence in situ hybridization using a specific 16S ribosomal RNA probe and genomic DNA probe. Seven patients (0.48%) presented mature B-cell lymphoma consisting of 6 DLBCL and 1 FL. An excess risk of DLBCL and FL was found in Q fever patients compared with the general population (SIR [95% confidence interval], 25.4 [11.4-56.4] and 6.7 [0.9-47.9], respectively). C burnetii was detected in CD68+ macrophages within both lymphoma and lymphadenitis tissues but localization in CD123+ plasmacytoid dendritic cells (pDCs) was found only in lymphoma tissues. Q fever patients with persistent focalized infection were found more at risk of lymphoma (hazard ratio, 9.35 [1.10-79.4]). Interleukin-10 (IL10) overproduction (P = .0003) was found in patients developing lymphoma. These results suggest that C burnetii should be added to the list of bacteria that promote human B-cell non-Hodgkin lymphoma, possibly by the infection of pDCs and IL10 overproduction. Screening for early lymphoma diagnosis should be considered in the management of patients with Q fever, especially those with persistent focalized infections.
Introduction
The incidence of non-Hodgkin lymphoma (NHL) is increasing in many regions making it a global health challenge for the coming years and is still associated with significant mortality.1,2 Risk factors include mainly infection, immunosuppression (HIV, organ-transplant recipients, high-dose chemotherapy, stem cell transplantation, and inherited immunodeficiency syndromes), or autoimmune diseases.1,3,4 Recent data from the InterLymph Consortium reported that some risk factors such as family history of NHL are common among NHL subtypes whereas others such as HIV or hepatitis C seropositivity appeared to be distinct among individuals or a few subtypes.4 Hereditary factors5 supporting a genetic predisposition include tumor necrosis factor (TNF) and interleukin-10 (IL10) polymorphisms,6 suggesting that these pro- or anti-inflammatory cytokines are critical in NHL physiopathology.4 Besides viruses, bacterial infections play a role in the development of some B-cell NHL, either by inhibition of immune function or by induction of chronic inflammatory response.1 Helicobacter pylori is associated with gastric B-cell lymphoma,7 Campylobacter jejuni with immunoproliferative small intestinal disease,8 Borrelia burgdorferi with cutaneous B-cell lymphoma,9 and Chlamydia psittaci with ocular adnexal lymphoma.10
Q fever is a zoonosis caused by the intracellular bacterium Coxiella burnetii.11 The primary infection, symptomatic in 10% to 60% of cases (then called acute Q fever), usually resolves spontaneously in a few weeks. In <5% of cases, the infection persists mainly as endocarditis or vascular infection.11 Persistent lymphadenitis has also been reported with detection of the bacterium in the excised lymph node (supplemental Table 1, see supplemental Data available on the Blood Web site).12-14 Although C burnetii is known to infect myeloid cells such as monocytes and macrophages,15 several lymphoid disorders have been reported in the course of Q fever, including mononucleosis syndrome, autoimmune manifestations, and monoclonal gammapathy.16-18 Moreover, 22 cases of lymphoproliferative disease, originating from B cells in all investigated cases, have been associated with Q fever19-22 (supplemental Table 2). Lymphoma, however, was previously considered to be a risk factor of persistent Q fever rather than a consequence of the infection.23,24
We previously reported a case of Q fever vascular infection revealed by an aortoenteric fistula.25 This patient, although improving under antibiotics, developed lymphadenitis near the site of infection, corresponding to a B-cell follicular lymphoma (FL). C burnetii was identified in the lymphoma tissue sample by fluorescent in situ hybridization (FISH). This index case prompted us to (1) collect cases of patients developing lymphoma after C burnetii primary infection in order to assess a possible excess risk of B-cell lymphoma in Q fever patients, (2) to test the presence of the bacterium in lymphoma biopsies, (3) to evaluate the IL10 production in Q fever patients with lymphoma, and (4) to investigate whether patients with persistent focalized infection were more at risk of lymphoma than acute Q fever patients.
Methods
French National Referral Center for Q Fever cohort
The French National Referral Center for Q Fever cohort database includes all patients with a possible or definite C burnetii infection diagnosed since 2004 in our center. Patients are included in the database if they are screened positive for C burnetii in case of specific demand or syndromic diagnosis (blood culture-negative endocarditis, pericarditis, uveitis, and central nervous system infection) and systematically on the following biopsies (osteoarticular samples, lymph nodes, skin samples, pharyngeal swabs, cardiac valve, and vascular samples). Moreover, C burnetii is also systematically sought for in all sera sent for Bartonella, Rickettsia, Francisella, Anaplasma, and Ehrlichia testing.
Our center receives over 10 000 samples for C burnetii testing each year26 from all over France and some other countries. The reasons for C burnetii testing include (1) initial screening test, (2) a confirmatory test after a positive initial local test, (3) suspicion of persistent infection, or (4) follow-up or discordance between clinical presentation and local serological results (supplemental Table 3). Clinical data are systematically collected for positive patients over the phone and added to our computerized database.
Diagnosis of C burnetii infection
Serology and molecular detection of C burnetii have been performed as previously described.27,28 Acute Q fever was defined by the association of clinical symptoms (fever, hepatitis, and/or pneumonia) with the serological criteria of a phase 2 immunoglobulin G (IgG) titer ≥200 and a phase 2 IgM titer ≥50,11 seroconversion or a positive polymerase chain reaction (PCR) and no endocarditis.29 Q fever endocarditis and vascular infection were defined according to recently updated criteria.30 Q fever lymphadenitis was defined as lymphadenitis associated with a serology consistent with acute Q fever (acute Q fever lymphadenitis) or with phase 1 IgG ≥800 or a positive test on lymph node (PCR, culture, immunohistochemistry, fluorescence in situ hybridization [FISH]).12-14 All atypical cases were discussed with an expert (D.R.) and included in the cohort only if a possible or definite C burnetii infection was retained.
Diagnosis of lymphoma
Formalin-fixed and paraffin-embedded biopsy samples were analyzed by an expert hematopathologist (L.X.) in all cases to confirm the diagnosis of lymphoma. Lymphoma specimens were diagnosed and typed according to World Health Organization criteria31 using morphologic examination and standard immunohistochemistry. The tissue sections were tested for Epstein-Barr virus (EBV) by FISH using the EBER probe (Dakopatts). FISH analysis was performed using BCL2, BCL6, and MYC break-apart probes as recommended by the manufacturer (VYSIS; Abbott).
Detection of C burnetii in lymphoma biopsy specimens
Immunofluorescence (IF), 16S ribosomal RNA (rRNA) FISH and genomic DNA FISH were performed as previously reported.32-34 For C burnetii–specific FISH, the probes CB-440 (5′-CTTGAGAATTTCTTCCCC-3′) and CB-1348 (5′-CACCGCGACATGCTGATTCGCG-3′) specifically target the C burnetii 16S rRNA sequences. We also used (1) EUB-338, which is complementary to a conserved region of the bacterial 16S rRNA molecule and specific for most Eubacteria,34 and (2) non-EUB-338 to exclude nonsense hybridization.32,33 Probes targeting the genomic sequences of C burnetii were generated by indirect labeling of the DNA with fluorescent dyes (ARES DNA labeling; Molecular Probes). The slides were blocked in 3% bovine serum albumin in phosphate-buffered saline–0.1% Tween 20 before IF using primary antibodies (supplemental Table 4) followed by goat anti-rabbit IgG or goat anti-mouse IgG conjugated to Alexa Fluor 555 or Alexa Fluor 647. The slides were rinsed with distilled water, air-dried, and mounted with the nucleic acid stain 4′,6-diamidino-2-phenylindole dihydrochloride (DAPI) from a ready-to-use solution, ProLong Gold Antifade Reagent (Molecular Probes). The imaging system was driven by Leica MetaMorph (version 1.6.0; Molecular Devices), and confocal analysis was performed using a Leica TCS 4DA confocal microscope. The method used to quantify the immune cell subset infected by C burnetii is detailed in the supplemental Methods. Twenty-two lymphoma samples from patients without Q fever were included as controls (supplemental Table 5).
Cytokine measurements
IL10 amount in sera was determined using specific immunoassays (R&D Systems) according to the manufacturer’s recommendations. IL10 and TNF were measured in supernatants from nonstimulated and C burnetii–stimulated peripheral blood mononuclear cells (PBMCs) as previously described.35
Statistical analysis
The incidence rate and its 95% confidence interval (CI) were computed for cases of diffuse large B-cell lymphoma (DLBCL) occurring during follow-up in the study population diagnosed between 2004 and 2014 (N = 1468) at the French National Reference Center of Q Fever. Patients were enrolled between January 2004 and December 2014, and they were followed up at most until the end of March 2015, which was used as censoring date. The person-years (PY) for the Q fever database were calculated as the total sum of the number of years between Q fever diagnosis of each patient and either his lymphoma diagnosis or his last available follow-up record or the censoring date. To confirm the significant elevation of the risk of DLBCL and FL in patients with Q fever compared with the French general population, we computed the standardized incidence ratios (SIRs)36 and their 95% CIs, taking into account the difference in age-sex distribution between the Q fever cohort and the general population. The data for the general population were extracted from the Francim National Registry reporting the estimation of the incidence of cancers in France in 2012.2 Comorbidities and immunodeficiency were not adjusted for as they were not mentioned in this report. A Cox proportional-hazards regression model was used to estimate the strength of the association between C burnetii–persistent focalized infection and occurrence of lymphoma. A sensitivity analysis was performed by removing from the data set those patients who experienced both acute Q fever and persistent focalized infections to verify the robustness of the results. Stata/SE 12.1 software (StataCorp LP) was used for these analyses.
Ethical considerations
The study was approved by the local ethics committee (Comité de Protection des Personnes Sud Mediterranée 1) under registration number 1355 and by the French National Drugs and Health Products Agency. All patients gave informed consent, in accordance with the Declaration of Helsinki.
Results
Index case
In August 2011, a 78-year-old man presented with Q fever abdominal aortic vascular infection complicated by an aortoenteric fistula.25 C burnetii was isolated from the resected vascular material by culture, PCR, and genome sequencing. Six months later, 18F fluorodeoxyglucose positron emission tomography (PET) identified a lateroaortic mass close to the infected focus with strong hypermetabolic uptake and several mesenteric lymph nodes. One year later, although the infection had improved clinically and serologically, these anomalies worsened (supplemental Figure 1I-III) such that a computed tomography scan-guided biopsy targeting the lateroaortic mass close to the vascular infected site (supplemental Figure 1II) identified a low-grade B-cell FL with FISH detection of C burnetii in CD68+ macrophages.
Frequency of lymphoma among Q fever patients
Between 2004 and 2014, 93 166 individuals were tested for C burnetii in our center; 91 628 individuals had negative serology and were not included in our cohort database (Figure 1). The age/sex distribution of these patients assessed but who were determined not to have Q fever was (mean ± standard deviation [SD]) 52.3 years (20.8 years) including 48 241 men (52.6%), respectively. Among the 1538 individuals from our cohort database, 70 were excluded after detailed medical records analysis (Figure 1) so that 1468 individuals were considered infected with a mean age of 50.50 ± 17.07 years and involving 998 men (68%). Patients infected were significantly younger (2-tailed t test, P < .0001) and more frequently male (2-tailed χ2 test, P < .0001) than patients assessed but who were determined not to have Q fever and not included in the parent database. Among the 1468 patients included, 1028 (70%) had an acute Q fever without progression to persistent focalized infection and 440 (30%) had a persistent focalized infection including 68 (4.6%) with initial acute Q fever. Among the 1468 Q fever patients, we found 7 patients (0.48%) including the index case with a diagnosis of lymphoma after C burnetii primary infection.
Study flowchart.aA patient with unexplained elevated serology was considered unexplored if he did not have transthoracic echocardiography (TTE), transesophageal echocardiography (TEE), and PET scan. bSeventy-six-year-old man with phase 1 IgG at 25 600 2 years after an acute Q fever living in an endemic area (La Rochelle, region of Poitou Charente). TTE and TEE were normal. PET scan was considered as normal by local practitioner. Second look by S.C. at our center found hyperfixation of subclavian arteries predominant right. Combination therapy with doxycycline and hydroxychloroquine resulted in a dramatic serology decrease. cOsteomyelitis of the pubic symphysis. dLung fibrosis (n = 5), lung pseudotumor, giant cell arteritis, chorioretinitis, and chronic hepatitis (1 case each).
Study flowchart.aA patient with unexplained elevated serology was considered unexplored if he did not have transthoracic echocardiography (TTE), transesophageal echocardiography (TEE), and PET scan. bSeventy-six-year-old man with phase 1 IgG at 25 600 2 years after an acute Q fever living in an endemic area (La Rochelle, region of Poitou Charente). TTE and TEE were normal. PET scan was considered as normal by local practitioner. Second look by S.C. at our center found hyperfixation of subclavian arteries predominant right. Combination therapy with doxycycline and hydroxychloroquine resulted in a dramatic serology decrease. cOsteomyelitis of the pubic symphysis. dLung fibrosis (n = 5), lung pseudotumor, giant cell arteritis, chorioretinitis, and chronic hepatitis (1 case each).
Increased incidence of DLBCL and FL in Q fever patients compared with the general population
Overall, DLBCL was found in 6 of the 1468 screened Q fever patients. The crude incidence rate (95% CI) was 280 (126-624) per 100 000 PY in Q fever patients, and 274 (103-730) for men and 294 (74-1177) for women, whereas the crude incidence rate/100 000 PY in the general population was 8 for men and 5 for women. The SIR (95% CI) was 25.4 (11.4-56.4) (20.3 [7.6-54.1] for men and 50.6 [12.6-202.2] for women but the difference was not significant). Only 1 patient included in our cohort was diagnosed with an FL, corresponding to a SIR (95% CI) of 6.7 (0.9-47.9).
Clinicopathological characteristics
Among the 7 patients analyzed, the mean age ± SD (range) at the time of Q fever diagnosis was 62.4 ± 11.6 (52-76) years and 5 patients (71%) were males. None of the patients were immunocompromised nor had a risk factor for lymphoma. Among the 7 reported patients, 4 were in the setting of endocarditis, 1 of vascular infection, 1 of acute Q fever, and 1 patient presented polyadenopathies. Clinical features including anatomical location and chronological history are summarized in Table 1 and supplemental Figure 2.
All 7 patients presented mature B-cell NHL,37 including 6 DLBCL (supplemental Figure 3) and 1 low-grade (1-2) FL. All tested patients presented elevated lactate dehydrogenase and β-2 microglobulin at lymphoma diagnosis. Ann Arbor staging at diagnosis was III in 2 patients and IV in 5 patients. Bcl-2 and Bcl-6 translocations were detected in 2 patients. EBV was negative in all lymphoma tissue samples. Details about DLBCL subtyping, and clinical treatment and outcome are provided in supplemental Methods and supplemental Figure 4, and supplemental Table 6, respectively.
C burnetii is present in both macrophages and plasmacytoid dendritic cells of lymphoma tumors
Presumably viable C burnetii was detected in 4 of the 7 patients with available tissue samples by all of the 3 fluorescent methods (IF, FISH targeting C burnetii 16S rRNA and FISH targeting C burnetii genomic DNA [supplemental Figure 5; supplemental Table 7]). In these 4 patients, the 3 fluorescent signals colocalized and demonstrated multiple rounded intracytoplasmic structures around the DAPI-stained nuclei (supplemental Figure 5a). Twenty-two control lymph node biopsies from patients without Q fever and presenting with various types of B-cell, T-cell, or Hodgkin lymphoma were treated in parallel experiments, and no specific signals were detected in the FISH/IF assays (supplemental Figure 5b; supplemental Table 5).
We investigated the cellular compartment harboring C burnetii using a combination of double staining and computerized microscopic analysis (supplemental Table 8). Because the distribution of the infected cells within an individual tumor was heterogeneous, the observation of infected cell subsets was focused on 2 distinct regions of lymphoma tissue with a high content of infected cells. C burnetii was found in 11% and 27% of CD68+ cells of each region, respectively (supplemental Figure 5c-d). In addition, C burnetii was present in 100% of CD123+ plasmacytoid dendritic cells (pDCs; supplemental Figure 5e). No C burnetii were detected in CD20+ lymphoma cells, CD3+ T cells, and S-100 protein+ dendritic cells (supplemental Figure 5f). Confocal microscopic analysis confirmed the presence of C burnetii using specific RNA probes inside the cytoplasmic vacuoles of infected cells (supplemental Videos 1-3). Among the 4 FISH-positive biopsies, PCR was negative in 2, immunohistochemistry in 4, and culture in 2. C burnetii was not detected by PCR (1 sample) or immunohistochemistry (4 samples) in the 3 FISH-negative samples.
As a control, we tested 3 patients with Q fever lymphadenitis but without lymphoma. C burnetii was detected in several CD68+ cells in 2 patients. In contrast to lymphoma samples, CD123+ pDCs cells were rare and none of them were infected (supplemental Figure 6). Altogether, these findings suggest a specific pDCs localization of C burnetii in the microenvironment of lymphomas occurring in the setting of C burnetii infection.
IL10 production in Q fever patients with lymphoma
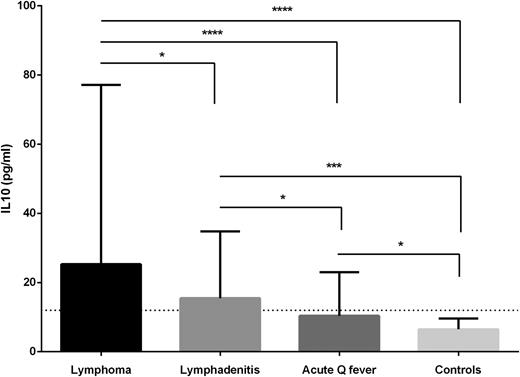
IL10 was tested as a putative instrumental cytokine in 386 sera from 79 patients and was found to be highly elevated in patients with lymphoma (median, 25.3 pg/mL [interquartile range, 17.7-77.1]) compared with patients with lymphadenitis (15.5 [8.8-34.8]), patients with acute Q fever without valvulopathy and no progression to persistent focalized infection (10.4 [7.3-23.0]) or healthy controls (6.1 [5.1-8.9], P < .05, 2-tailed Kruskal-Wallis test with Dunn multiple comparisons test, Figure 2; supplemental Figure 7). Among Q fever patients, those with lymphoma have very significantly higher IL10 levels than those without lymphoma (25.3 [17.7-77.1] vs 12.7 [8.1-32.9], P = .0003, 2-tailed Mann-Whitney test). IL10 production by nonstimulated PBMCs was highly increased in patients with lymphoma whereas TNF production was very low even after stimulation by C burnetii (P < .05, supplemental Figure 8).
IL10 levels in Q fever patients. IL10 was evaluated in 397 sera including 24 sera from 5 patients with lymphoma, 250 sera from 48 patients with lymphadenitis, 112 sera from 26 patients with acute Q fever without valvulopathy and without progression to persistent focalized infection and 11 healthy controls. Median and interquartile range. The horizontal line corresponds to the maximum level observed in 11 healthy controls (12 pg/mL). *P < .05, ***P < .0005, ****P < .00005. Bilateral Mann-Whitney test. A gradient was observed suggesting that patients with lymphadenitis without lymphoma may represent a prelymphoma condition.
IL10 levels in Q fever patients. IL10 was evaluated in 397 sera including 24 sera from 5 patients with lymphoma, 250 sera from 48 patients with lymphadenitis, 112 sera from 26 patients with acute Q fever without valvulopathy and without progression to persistent focalized infection and 11 healthy controls. Median and interquartile range. The horizontal line corresponds to the maximum level observed in 11 healthy controls (12 pg/mL). *P < .05, ***P < .0005, ****P < .00005. Bilateral Mann-Whitney test. A gradient was observed suggesting that patients with lymphadenitis without lymphoma may represent a prelymphoma condition.
Risk factors of lymphoma among Q fever patients
Comparing Q fever patients with lymphoma, patients with lymphadenitis who did not develop lymphoma, and other Q fever patients (Table 2), we found that persistent focalized infection was a risk factor for lymphadenitis (odds ratio [OR], 1.78; 95% CI, 1.04-3.03; P = .047) but to a lesser extent than for lymphoma (OR, 14.54; 95% CI, 2.14-337.7; P = .007). Indeed, patients with persistent focalized infections were more likely to develop lymphoma (6 of 440 [1.4%]) than acute Q fever patients without known progression to persistent focalized infection (1 of 1028 [0.1%]; 2-tailed Fisher exact test; P = .007). This was confirmed by a Cox model (hazard ratio [HR], 9.35; 95% CI, 1.10-79.4; P = .041) and after the sensitivity analysis excluding patients with acute Q fever progressing to a persistent focalized infection (HR, 9.41; 95% CI, 1.07-82.9; P = .043).
Discussion
In this study, we found 7 occurrences of B-cell NHL after C burnetii primary infection in patients included in the 2004 to 2014 French National Referral Center for Q Fever cohort database. The strength of this association was suggested by a 25-fold excess risk of DLBCL in Q fever patients compared with the general population in France. The inclusion period of our study (2004-2014) was consistent with that of the French registry used for comparison (2012).2 This size effect is unlikely to be attributable to plausible confounding38 as it was adjusted for age and sex, and is far greater than the mean annual increase incidence (3%) in the French general population between 2004 and 2014.2 This excess risk was consistent with other bacterium-related cancers.39
The detection of the bacterium in lymphoma and lymphadenitis biopsies was ascertained by 3 different techniques (16S rRNA FISH, genomic DNA FISH, and IF) with confocal microscopy validation. Several negative controls confirmed the specificity of our technique (supplemental Table 5). Moreover, presumably viable C burnetii were observed in the cytoplasm of macrophages in multiple rounded structures consistent with intracytoplasmic vacuoles typical of the C burnetii infection.11 The very high sensitivity of fluorescence-based techniques (IF and FISH) was critical in the detection of the bacterium as all other techniques were negative. The use of a universal eubacterial probe for FISH detection (Eub-338)40,41 allowed us to exclude other infectious etiologies in biopsy samples, such as H pylori, C jejuni or B burgdorferi, because all Eub-338–positive signals colocalized with C burnetii–specific FISH.
The specific pDC infection and increased IL10 levels in patients with Q fever–associated lymphoma suggest an alteration of the immune signals within the lymphoma microenvironment. IL10 was previously shown to be a B-cell growth factor with immunosuppressive properties that upregulates bcl-2 expression.6 Viral IL10 is critical for B-cell transformation and proliferation by EBV.42,43 High IL10 levels were found in the sera of NHL patients44-47 and were associated with poor prognosis.44,45,48-51 In Q fever, overproduction of IL10 by infected monocytes is critical in sustaining replication of the bacterium, and is associated with an inhibition of the microbicidal activity of macrophages.52 IL10 serum levels are increased in acute Q fever patients with valvulopathy and in endocarditis, and persistent elevated IL10 levels are predictive of relapse.52
A putative scenario could be proposed in which C burnetii–infected monocytes and pDCs induce impairment of the immune system favoring lymphoma occurrence. In primary or persistent Q fever, infected monocytes migrate via lymphatic vessels to the lymph nodes. The influence of the lymph node microenvironment, including apoptotic lymphocytes from germinal centers, may induce a specific polarization of the monocytes/macrophages toward an M2 profile, characterized by a higher production of anti-inflammatory cytokines and IL10.53 Our observation of intermediate IL10 levels in C burnetii–related lymphadenitis, which occurs more frequently in the setting of persistent infection, suggests that it might represent a prelymphoma condition. Infection of pDCs may represent a critical step toward lymphomagenesis as these cells were not infected in patients with lymphadenitis. Infected pDCs can also induce an increase in IL10 secretion via type 1 interferon production54 and upregulation of inducible costimulating ligand expression.55 Thus, monocytes and pDCs are probably both responsible for the impairment of the immune system. IL10 in turn stimulates the replication of C burnetii in monocytes56 and prevents apoptosis of germinal center B cells by overexpression of the Bcl-2 protein.57 IL10-mediated immune impairment may be favorable to both C burnetii replication and lymphoma growth. Our observation of bcl-2 and bcl-6 rearrangements suggests that the early steps of lymphomagenesis may be shared in Q fever patients and in the general population. This hypothesis is also consistent with the localization of C burnetii in the microenvironment but not in the neoplastic cells. Of note, the presence of C burnetii in lymphoma tissues cannot be explained by a clinical setting of immunosuppression in our patients. The absence of EBV in C burnetii–infected tumors also argues against a preexisting immunosuppression that could have favored C burnetii persistence in the tumor microenvironment.
The InterLymph consortium reported that risk factors of lymphoid neoplasms are related to either specific NHL subtypes or to virtually all lymphomas.4,37 Considering the 7 included cases (Table 1) and 4 additional cases from France, Spain, and Israel (supplemental Table 6), we found 6 DLBCL, 2 FL, 2 marginal zone lymphomas, and 1 lymphoplasmocytic lymphoma. In addition, at least 5 hairy cell leukemias have been reported in the literature19,21,22 (supplemental Table 2). Altogether, the 16 clinical cases of Q fever–associated lymphoproliferative lesions reported in the present work and in the literature correspond to mature B-cell neoplasms.37 Larger series are needed to confirm that the risk of lymphoma occurrence in Q fever patients does not involve precursor lymphoid neoplasms nor T-cell lymphomas.
General farm worker was identified by the InterLymph Consortium as an independent risk factor affecting overall NHL risk4 and particularly hairy cell leukemia.58 On the other hand, occupation as a farmer is the main risk factor for Q fever, associated with hairy cell leukemia in the literature.19,21,22 Altogether, these findings suggest that C burnetii infection could be responsible, at least in part, for the excess risk of NHL in farm workers. Although our data are supportive of an association between C burnetii and B-cell NHL, other lymphoma risk factors could not be excluded. Indeed, this was a retrospective study and some factors like family history of hematologic malignancy were not collected.4 Future prospective studies are needed to confirm the accurate incidence rate, as our cohort possibly suffered from center bias that probably tends to overestimate incidence. Conversely, the incidence may have been underestimated as the Q fever–lymphoma association was unknown, so that some lymphoma cases possibly diagnosed several months to years after Q fever may have been missed.
For the first time in the literature, we provide evidence that C burnetii may be a lymphoma cofactor. This confirms other abundant data linking this bacterium to lymphoproliferative disorders (supplemental Table 2), including very recent reports from the Dutch outbreak.22 Although we cannot conclude that Q fever directly causes lymphoma, our results are unlikely to be due to chance because several criteria for causation are fulfilled.38,59 Additional reports of cases treated by antibiotics alone would provide greater support for the purported association. Disregarding the causality issue, the link between Q fever and lymphoma that we evidence herein should not be neglected because early diagnosis of lymphoma would result in improved outcomes of Q fever patients.1 Moreover, the management of patients with B-cell NHL would be improved by the detection of C burnetii infection in endemic areas.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors acknowledge Dr Jose M Miro (Infectious Diseases), Dr Olga Balaguer (Pathology), Dr Isabel Sanfeliu (Microbiology), and Dr Julio Delgado (Hematology) for providing accurate data about the Spanish case; Dr Vincent Poindron (Internal Medicine) for providing accurate data about the patient 2; Dr Mathilde Versini (Internal Medicine) for providing data about a new case diagnosed during the revision of this manuscript (additional patient 11 mentioned in supplemental Table 6); and Romain Verdet for supplemental Figure 2. The authors thank Amira Ben Amara for all IL10 analysis and Karolina Griffiths for English reviewing.
This study was supported by the French National Referral Center for Q fever.
Authorship
Contribution: C.M. and M.M. wrote the article; G.A. performed the FISH analysis and wrote the article; A.G., H.D., M.D., and A.M. provided clinical information; G.R. made the challenging diagnostic biopsy of the index case (13 mm from the aortic wall!); S.C. performed the PET scan of the index patient; M.P.C. and C.P. performed the statistical analysis; P.R. determined the genetic signatures of the lymphoma biopsy samples; H.L. performed immunohistochemistry on the lymphoma biopsy samples; B.N. participated in the discussion; J.-L.M. participated in dendritic cell investigation, analysis of the results, and in the discussion; L.X. performed histopathological analysis of all biopsy samples, wrote the article, and participated in the discussion; D.R. designed and supervised the study; and C.M. and M.M. had full access to all data in the study and take responsibility for the integrity of the data and the accuracy of its analysis.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Didier Raoult, Aix-Marseille Université, Unité de Recherche sur les Maladies Infectieuses et Tropicales Emergentes, Faculté de Médecine, 27 Bd Jean Moulin, 13005 Marseille, France; e-mail: didier.raoult@gmail.com; and Matthieu Million, Faculté de Médecine, Unité de Recherche sur les Maladies Infectieuses et Tropicales Emergentes, 27 boulevard Jean Moulin, 13005 Marseille, France; e-mail: matthieumillion@gmail.com.
References
Author notes
C.M. and M.M. are equal coauthors.