Key Points
This study demonstrates a novel mechanistic role for a specific N-linked glycan in regulating PAR1 proteolysis.
We provide the first description of an APC variant with enhanced therapeutic cytoprotective activity in vivo.
Abstract
Activated protein C (APC) is an anticoagulant protease that initiates cell signaling via protease-activated receptor 1 (PAR1) to regulate vascular integrity and inflammatory response. In this study, a recombinant APC variant (APCN329Q) mimicking the naturally occurring APC-β plasma glycoform was found to exhibit superior PAR1 proteolysis at a cleavage site that selectively mediates cytoprotective signaling. APCN329Q also enhanced integrin αMβ2-dependent PAR1 proteolysis to exert significantly improved antiinflammatory activity on macrophages compared with wild-type APC. Recent therapeutic applications of recombinant APC in ischemic stroke models have used APC variants with limited anticoagulant activity to negate potential bleeding side effects. Using a mouse model of ischemic stroke and late t-PA intervention, the neuroprotective activity of a murine APC variant with limited anticoagulant activity (mAPCPS) was compared with an identical APC variant except for the absence of glycosylation at the APC-β sequon (mAPCPS/N329Q). Remarkably, mAPCPS/N329Q limited cerebral ischemic injury and reduced brain lesion volume significantly more effectively than mAPCPS. Collectively, this study reveals the importance of APC glycosylation in controlling the efficacy of PAR1 proteolysis by APC and demonstrates the potential of novel APC variants with superior cytoprotective signaling function as enhanced therapeutic agents for the treatment of ischemic stroke.
Introduction
Activated protein C (APC) plays multiple regulatory roles that cumulatively serve to limit thrombus development1 and attenuate inflammation.2 APC engagement with the endothelial cell protein C receptor (EPCR) facilitates proteolysis-dependent activation of protease-activated receptor 1 (PAR1),3 which protects the endothelial cell barrier from disruptive agents,4 inhibits apoptosis,5,6 and limits cytokine expression from cells exposed to proinflammatory stimuli.6,7
Recombinant APC signaling attenuates neurovascular sequelae associated with experimental murine ischemic stroke8-10 and limits neurotoxicity caused by subsequent thrombolytic tissue plasminogen activator (t-PA) administration.11,12 Recombinant APC therefore represents a promising neuroprotective drug candidate to treat ischemic stroke, and a recombinant nonanticoagulant APC variant is currently being evaluated as an adjunctive therapy to minimize the adverse effects of t-PA administration.13,14
A recombinant APC variant (APCN329Q) that mimics the glycosylation pattern of the endogenous plasma APC-β glycoform15 exhibits significantly enhanced PAR1-dependent cytoprotective activity on endothelial cells compared with wild-type APC.16 In this study, we sought to determine the molecular basis for superior APC-β cytoprotective signaling and establish whether ablation of the APC-β glycosylation sequon to accelerate cytoprotective signaling represents a novel means by which to increase the therapeutic efficacy of APC in an ischemic stroke model.
Study design
Materials
A complete description of the reagents used in this study is included in the supplemental Material, found on the Blood Web site.
Preparation of recombinant APC
Detection of PAR1 proteolysis on HEK 293T cells
PAR1 reporter–transfected 293T cells were incubated with APC in serum-free Dulbecco’s modified Eagle medium supplemented with 3 mM CaCl2 and 0.6 mM MgCl2 for 3 hours, after which the supernatant was removed and alkaline phosphatase (AP) activity measured using an AP substrate (QUANTI-Blue, Invivogen) at 650 nm.
APC inhibition of cytokine secretion from LPS-stimulated macrophages
Analysis of APC inhibition of cytokine secretion from RAW264.7 cells was performed as previously described.18 Tumor necrosis factor-alpha (TNFα) and IL-6 were measured by enyme-linked immunosorbent assay (ELISA) (R&D Systems).
Mouse model of ischemic stroke
Ischemic stroke in mice was induced as previously described19 and is further detailed in the supplemental Material. Briefly, 200 µL of 10 mg/kg t-PA (10% bolus, 90% perfusion during 20 minutes) with or without 0.2 mg/kg mAPCPS or mAPCPS/N329Q (50% bolus, 50% perfusion during 20 minutes) were given intravenously 3 hours after vessel occlusion. After 24 hours, mice were killed and their brains removed and frozen in isopentane. 20 μm cryostat-cut coronal brain sections were stained with thionine and analyzed (ImageJ 1.48V). For volume analysis, one section of every 10 was stained covering the entire lesion, and lesion (unstained) areas were measured.
Results and discussion
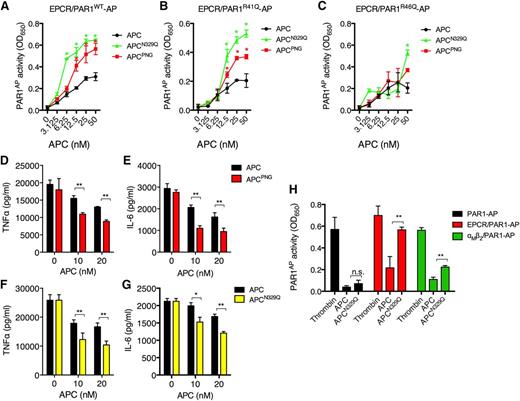
We hypothesized that enhanced cytoprotective PAR1 signaling by APC-β was a consequence of accelerated EPCR-dependent PAR1 proteolysis. To test this, 293T cells cotransfected with EPCR and PAR1 linked to an N-terminal alkaline phosphatase (AP) cleavage reporter20 were treated with recombinant APC-β (APCN329Q) or N-glycosidase PNGase F–treated APC (APCPNG), in which all N-linked glycans were excised.16 Interestingly, both APCPNG and APCN329Q demonstrated up to a fourfold increased PAR1 proteolysis when compared with wild-type APC (Figure 1A) or other APC glycoform variants (data not shown).
APC glycosylation at Asn-329 specifically restricts PAR1 proteolysis and signaling activity. 293T cells transfected with EPCR and either (A) PAR1-AP, (B) PAR1R41Q-AP, (C) PAR1R46Q-AP were treated with APC (black circles), APCPNG (red squares) or APCN329Q (green triangles) (all 6.25-50 nM) for 3 hours, before AP activity was measured using an AP substrate. An extended period of incubation with the AP substrate was required to observe any AP activity in cell supernatants from APC-treated EPCR/PAR1R46Q-AP–transfected 293T cells. Murine macrophages (RAW264.7) were incubated with APC (black bars) or APCPNG (red bars) (5-20 nM) for 3 hours before stimulation with LPS (50 ng/mL) for 18 hours. (D) TNFα and (E) IL-6 secretion were measured by ELISA. Similarly, APC (black bars) and APCN329Q (yellow bars; 10-20 nM) were incubated with RAW264.7 macrophages for 3 hours before LPS (50 ng/mL) incubation for 18 hours. The resultant supernatants were assessed for the presence of (F) TNFα and (G) IL-6 as before. 293T-transfected cells with (H) PAR1-AP alone (black), EPCR/PAR1-AP (red), or αMβ2/PAR1-AP (green) were treated with thrombin (5 nM), wild-type APC, or APCN329Q (both 50 nM) for 3 hours before AP activity was assessed. All experiments were performed at least in triplicate and results are presented as the mean ± standard error of the mean; *P < .05, **P < .01 by Student t test.
APC glycosylation at Asn-329 specifically restricts PAR1 proteolysis and signaling activity. 293T cells transfected with EPCR and either (A) PAR1-AP, (B) PAR1R41Q-AP, (C) PAR1R46Q-AP were treated with APC (black circles), APCPNG (red squares) or APCN329Q (green triangles) (all 6.25-50 nM) for 3 hours, before AP activity was measured using an AP substrate. An extended period of incubation with the AP substrate was required to observe any AP activity in cell supernatants from APC-treated EPCR/PAR1R46Q-AP–transfected 293T cells. Murine macrophages (RAW264.7) were incubated with APC (black bars) or APCPNG (red bars) (5-20 nM) for 3 hours before stimulation with LPS (50 ng/mL) for 18 hours. (D) TNFα and (E) IL-6 secretion were measured by ELISA. Similarly, APC (black bars) and APCN329Q (yellow bars; 10-20 nM) were incubated with RAW264.7 macrophages for 3 hours before LPS (50 ng/mL) incubation for 18 hours. The resultant supernatants were assessed for the presence of (F) TNFα and (G) IL-6 as before. 293T-transfected cells with (H) PAR1-AP alone (black), EPCR/PAR1-AP (red), or αMβ2/PAR1-AP (green) were treated with thrombin (5 nM), wild-type APC, or APCN329Q (both 50 nM) for 3 hours before AP activity was assessed. All experiments were performed at least in triplicate and results are presented as the mean ± standard error of the mean; *P < .05, **P < .01 by Student t test.
Generation of unique tethered ligands by APC or thrombin, caused by distinct cleavage events at Arg-46 or Arg-41, respectively, on PAR1, has been proposed as a potential mechanism to explain the disparate outcomes of PAR1 signaling initiated by each protease.21,22 To determine whether the enhanced cytoprotective activity exhibited by APCN329Q was a consequence of enhanced PAR1 proteolysis at the noncanonical Arg-46 cleavage site, 293T cells were cotransfected with EPCR and either PAR1R41Q-AP or PAR1R46Q-AP reporter variants (Figure 1B-C). Notably, cleavage at the Arg-46 site on PAR1 was especially sensitive to the loss of N-linked glycosylation after PNGase F treatment, and particularly loss of glycosylation at the APC-β sequon (Figure 1B). In contrast, Arg-41 proteolysis was limited in the presence of APC and largely insensitive to glycosylation status (Figure 1C). The increased rate of Arg-46 proteolysis on PAR1 by APCPNG and APCN329Q provides a novel molecular explanation for the enhanced cytoprotective activity of APCN329Q on endothelial cells and supports the emerging concept of biased PAR1 agonism to achieve downstream cytoprotective signaling.
αMβ2 can replace EPCR as a coreceptor for PAR1-dependent APC antiinflammatory signaling on macrophages.23 To determine whether APC glycosylation also negatively regulates αMβ2-dependent PAR1 signaling, we tested the ability of APCPNG and APCN329Q to attenuate proinflammatory cytokine release from lipopolysaccharide (LPS)-stimulated macrophages. APCPNG and APCN329Q reduced TNFα and IL-6 release from LPS-treated macrophages significantly more effectively than wild-type APC (Figure 1D-G). 293T cells coexpressing recombinant human αMβ2 and PAR1-AP were used to determine whether enhanced antiinflammatory activity was mediated by increased αMβ2-dependent PAR1 proteolysis by APCN329Q. Interestingly, the αM subunit mediated all coreceptor activity for αMβ2-PAR1 proteolysis by APC, and enabled APC cleavage of PAR1 at both canonical (Arg-41) and noncanonical (Arg-46) sites (supplemental Figure 1). Both APCPNG and APCN329Q significantly enhanced αMβ2-dependent PAR1 proteolysis (Figure 1H), indicating that N-linked glycans at Asn-329 regulate cytoprotective signaling by APC by impairing PAR1 proteolysis, irrespective of the APC coreceptor used.
Thrombolysis with t-PA is the only Food and Drug Administration–approved thrombolytic treatment of ischemic stroke, but can only be safely administered within 3 hours of symptom onset because of significant neurotoxicity.24 Recombinant APC variants with severely attenuated anticoagulant activity reduce t-PA–associated toxicity in murine models of ischemic stroke in a PAR1-dependent manner.11,12 We hypothesized that a recombinant nonanticoagulant APC that consists solely in the β-glycoform would be more efficacious in reducing t-PA neurotoxicity than existing APC variants in a murine model of ischemic stroke with late t-PA intervention. Similar to its human equivalent, in vitro analysis indicated that loss of N-linked glycan attachment at Asn-329 in murine APC (mAPCN329Q) enhanced PAR1 proteolytic and antiinflammatory activity (Figure 2A-C). To determine whether the therapeutic activity of an APC variant with reduced anticoagulant activity could be enhanced by selective de-glycosylation, a signaling-competent mAPC variant (mAPC-D36A/L38D/A39V; mAPCPS) with severely impaired anticoagulant activity in murine plasma25 was prepared. The therapeutic efficacy of this variant was compared with an identical mAPC variant prepared on an APC-β background (mAPCPS/N329Q) (Figure 2D-E). As anticipated, sham-operated mice did not develop cerebral injury (supplemental Figure 2). Like other mAPC variants with reduced anticoagulant activity,12 mAPCPS retained the ability to reduce t-PA–induced cerebral injury 24 hours after stroke and t-PA administration, albeit modestly in this model (Figure 2F-G). mAPCPS/N329Q, however, significantly reduced brain lesion size compared with when t-PA was administered alone after the ischemic event (P = .006; Figure 2F-G). Remarkably, mAPCPS/N329Q was also significantly superior in preventing brain lesions compared with mAPCPS (P = .004; Figure 2F-G), despite their structures only differing by the presence or absence of a specific N-linked glycan.
APC-β exhibits superior inhibition of cerebral injury in murine ischemic stroke. (A) 293T cells transfected with EPCR and PAR1-AP were incubated with mAPC or mAPCN329Q (6.25-50 nM) for 3 hours. AP activity was assessed using QUANTI Blue AP substrate, as before. RAW264.7 macrophages were incubated with mAPC (black bars) or mAPCN329Q (red bars) (12.5-50 nM) for 3 hours before stimulation with LPS (50 ng/mL) for 18 hours. Secretion of (B) TNFα and (C) IL-6 was measured by ELISA. (D) Schematic description of the ischemic stroke model and study protocol. (E) Sodium dodecyl sulfate polyacrylamide gel electrophoresis analysis under reducing conditions of purified recombinant mAPCPS and mAPCPS/N329Q. Proteins were stained with Coomassie Brilliant Blue. The heavy chain of mAPCPS migrates as a diffuse band composed of the APC-α heavy chain and APC-β glycoform heavy chain fractions. The heavy chain of mAPCPS/N329Q is composed solely of the APC-β fraction. (F) Brain infarct volumes post-stroke and t-PA administration were calculated in each mouse by staining 20-μm coronal brain sections with thionine, which is unable to stain lesion areas. For volume analysis, 1 section of every 10 covering the entire lesion was analyzed. Individual values and the median of each group are represented; n = 9-15; *P = .006, **P = .004. (G) Images corresponding to 2 thionine-stained sections of representative mice from each group are shown. Lesion areas are unstained, whereas remaining healthy tissue is stained purple.
APC-β exhibits superior inhibition of cerebral injury in murine ischemic stroke. (A) 293T cells transfected with EPCR and PAR1-AP were incubated with mAPC or mAPCN329Q (6.25-50 nM) for 3 hours. AP activity was assessed using QUANTI Blue AP substrate, as before. RAW264.7 macrophages were incubated with mAPC (black bars) or mAPCN329Q (red bars) (12.5-50 nM) for 3 hours before stimulation with LPS (50 ng/mL) for 18 hours. Secretion of (B) TNFα and (C) IL-6 was measured by ELISA. (D) Schematic description of the ischemic stroke model and study protocol. (E) Sodium dodecyl sulfate polyacrylamide gel electrophoresis analysis under reducing conditions of purified recombinant mAPCPS and mAPCPS/N329Q. Proteins were stained with Coomassie Brilliant Blue. The heavy chain of mAPCPS migrates as a diffuse band composed of the APC-α heavy chain and APC-β glycoform heavy chain fractions. The heavy chain of mAPCPS/N329Q is composed solely of the APC-β fraction. (F) Brain infarct volumes post-stroke and t-PA administration were calculated in each mouse by staining 20-μm coronal brain sections with thionine, which is unable to stain lesion areas. For volume analysis, 1 section of every 10 covering the entire lesion was analyzed. Individual values and the median of each group are represented; n = 9-15; *P = .006, **P = .004. (G) Images corresponding to 2 thionine-stained sections of representative mice from each group are shown. Lesion areas are unstained, whereas remaining healthy tissue is stained purple.
This study shows that effective cytoprotective PAR1 signaling by APC is determined by specific N-linked glycans absent in certain naturally occurring APC glycoforms, revealing new mechanistic insights as to how PAR1 proteolysis by APC is regulated. Moreover, APC-β sequon disruption boosts recombinant nonanticoagulant APC amelioration of cerebral ischemic injury after late t-PA treatment in ischemic stroke. Although further studies are required to investigate which of the multiple PAR1-dependent neuroprotective functions mediated by APC in vivo13 are particularly enhanced by APCPS/N329Q in this setting, recombinant APC variants capable of hyperactive cytoprotective signaling represent a novel approach to boost the efficacy of APC-based therapeutic strategies without increasing dosage or compromising safety.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors acknowledge Eva Molina and Maider Esparza for their excellent technical assistance.
This study was funded by a Science Foundation Ireland Starting Investigator Research Grant (09/SIRG/B1590) (R.J.S.P.), the National Children’s Research Centre (R.J.S.P.), a European Research Area Network (ERANET)-Neuron grant (PRI-PIMNEU-2011-1334), and the Instituto de Salud Carlos III (PI10/01432, PI13/00072, PI11/1458, RECAVA RD06/0014/0008).
Authorship
Contribution: E.M.G., M.G.D., A.S., L.M.Q., C.D., and S.E.R., performed experiments and analyzed data; P.T.W., J.O., J.H., O.P.S., and J.S.O’D. analyzed data and planned experiments; R.M. and R.J.S.P. analyzed data, planned experiments, and conceived the study; and all authors contributed to the preparation of the manuscript.
Conflict-of-interest disclosure: R.J.S.P. has received honoraria from Octapharma and research funding from Bayer Healthcare Pharmaceuticals.
Correspondence: Roger J. S. Preston, School of Medicine, Trinity College Dublin, United Kingdom; e-mail: prestonr@tcd.ie; and Ramón Montes, Center for Applied Medical Research, University of Navarra, Pamplona, Spain; e-mail: montesdiazpamplona@outlook.com.
References
Author notes
E.M.G. and M.G.D. contributed equally to this study.