Key Points
COMP negatively regulates hemostasis and thrombosis.
COMP is a natural inhibitor of thrombin.
Abstract
Thrombin is an effector enzyme for hemostasis and thrombosis; however, endogenous regulators of thrombin remain elusive. Cartilage oligomeric matrix protein (COMP), a matricellular protein also known as thrombospondin-5, is essential for maintaining vascular homeostasis. Here, we asked whether COMP is involved in the process of blood coagulation. COMP deficiency shortened tail-bleeding and clotting time and accelerated ferric-chloride–induced thrombosis in mice. The absence of COMP had no effect on platelet count. In contrast, COMP specifically inhibited thrombin-induced platelet aggregation, activation, and retraction and the thrombin-mediated cleavage of fibrinogen. Furthermore, surface plasmon resonance analysis revealed direct thrombin-COMP binding (KD = 1.38 ± 0.24 μM). In particular, blockage of thrombin exosites with compounds specific for exosite I (hirudin and HD1 aptamer) or exosite II (heparin and HD22 aptamer) impaired the COMP-thrombin interaction, indicating a 2-site binding mechanism. Additionally, epidermal growth factor–like repeats (amino acids 84-261) were identified as a COMP binding site for thrombin. Moreover, COMP was expressed in and secreted by platelets. Using bone marrow transplantation and platelet transfusion to create chimeric mice, platelet-derived but not vessel-wall–derived COMP was demonstrated to inhibit coagulation. Taken together, COMP is an endogenous thrombin inhibitor and negative regulator of hemostasis and thrombosis.
Introduction
The serine protease thrombin is the central enzyme in the coagulation cascade. This protein plays a critical role in both hemostasis and thrombosis via the cleavage of fibrinogen to fibrin, the activation of platelets, and the conversion of procofactors to active cofactors.1 Thrombin is therefore a viable target for anticoagulation. Exogenous thrombin inhibitors are generally found in the saliva of hematophagous animals. The most characterized inhibitor is hirudin, a 65-reside protein/peptide that binds to thrombin with high affinity.2 In addition, endogenous thrombin inhibitors also exist so that thrombin does not occlude blood flow and cause thrombosis. The currently known endogenous thrombin inhibitors, including antithrombin, heparin cofactor II, protein C inhibitor, and protease nexin 1, belong to the serpin family.3 Exploring the novel regulatory mechanisms of thrombin will shed light on the prevention and treatment of cardiovascular and thrombus diseases including stroke, atherothrombosis, and deep vein thrombosis.
Cartilage oligomeric matrix protein (COMP), a 524-kDa pentameric noncollagenous glycoprotein, belongs to the thrombospondin (TSP) family and is also known as TSP-5.4,5 TSPs consist of 5 homologous members including TSP-1 and -2, which are trimers, and TSP-3, -4, and -5, which are pentamers.5,6 Recent information regarding the function of TSPs has placed this protein family among the most active and potent regulators of homeostasis and pathologies. For example, TSP-1, the most characterized TSP member, is present in the extracellular matrix, circulates in plasma at low concentrations, and is stored in platelet α-granules, where it is released upon activation.7 TSP-1 potentially interacts with several platelet receptors including the integrins αvβ3 and αIIbβ3, CD36, and CD47 and regulates platelet adhesion, activation, and aggregation.8,9 TSP-2 is required by megakaryocytes for normal platelet formation and function.10 A genetic association study has revealed coronary artery disease and myocardial infarction–associated single-nucleotide polymorphisms in the TSP-1, -2, and -4 genes.11 COMP (TSP-5) is expressed in all types of cartilage, the vitreous of the eye, tendons, heart, and vascular smooth muscle cells. Compelling evidence has indicated an essential role for COMP in cartilage and bone metabolism and involvement in arthritis.12 Our recent studies have also revealed that COMP is a central player in maintaining homeostasis in the cardiovascular system. COMP retains the contractile phenotype of vascular smooth muscle cells and prevents postinjury vascular neointima formation and vascular calcification.13,14 COMP deficiency renders spontaneous dilated cardiomyopathy and heart failure in mice.15 ADAMTS-7, the only COMP-degrading enzyme identified in vessels,16 has been recently reported in a genome-wide study to be significantly associated with coronary artery disease in humans, and it promotes vascular stenosis and calcification in rodents.17-19 However, whether COMP affects the coagulation cascade remains unknown. In this study, we explored the potential role of COMP in the regulation of hemostasis and thrombosis.
Methods
Animal preparation
All studies followed the guidelines of the animal care and use committee of Peking University. COMP−/− mice in the C57/BL6 background strain were kindly provided by Professor Oldberg Ake from the Department of Cell and Molecular Biology at Lund University, Sweden.20 COMP+/+ littermates as wild-type (WT) and COMP−/− mice were used for experiments. Animals were genotyped using polymerase chain reaction via respective primers (supplemental Table 1 available at the Blood Web site).
Ferric chloride–induced carotid artery injury
Ferric chloride (FeCl3)-induced carotid artery injury was performed as previously described with some modifications.21 Briefly, 8-week-old male mice were anesthetized, and the right common carotid artery was exposed. A miniature Doppler flow probe (model 0.5VB; Transonic Systems, Ithaca, NY) was positioned around the artery, and a 1 × 2-mm2 strip of 1M Whatman filter paper (Whatman International) soaked in 20% FeCl3 was applied to the adventitia of the artery for 3 min. The filter paper was then removed, and thrombus formation in the artery was monitored via the blood flow rate until complete occlusion (flow rate = 0 mL/min).
Radiation chimeras
Bone marrow transplantation was performed based on previous reports with minor modifications.22,23 Briefly, mice were exposed to γ-irradiation from a 60Co source (Department of Applied Chemistry, Peking University) followed by the injection of bone marrow cells (5 × 106 cells/mice) via the tail vein. At 4 weeks posttransplantation, tail bleeding time, blood clotting time, and FeCl3-induced thrombosis of the carotid artery were assessed using the reconstituted mice as described above.
Adoptive platelet transfusion
Washed mouse platelets were isolated from WT and COMP−/− mice and then resuspended in the serum of COMP−/− mice at 4 × 109 platelets/mL. Approximately 0.8 × 109 platelets (200 μL) were injected into 1 COMP−/− mouse via the lateral tail vein.24 One hour after platelet transfusion, mouse tail-bleeding time, clotting time, and FeCl3-induced carotid artery injury were measured.
Statistical analysis
Statistical analyses involved the use of GraphPad Prism 5.0 (GraphPad Software, La Jolla, CA). All data are presented as the mean ± standard error of the mean. Comparisons of tail-bleeding time, clotting time, and FeCl3-induced thrombosis time in mice were analyzed using nonparametric tests (the Mann-Whitney test for 2 groups and the Kruskal-Wallis test followed by the Dunn test for >2 groups). The unpaired Student t test (2 sided) was applied for the rest of the comparisons between 2 groups. Comparisons among more than 2 groups were made with a 1-way analysis of variance followed by the Student-Newman-Keuls test for post hoc comparison or a 2-way analysis of variance followed by the Bonferroni test. A P < .05 was considered statistically significant.
More details regarding materials and methods are available in supplemental Methods.
Results
COMP−/− mice exhibited enhanced procoagulant activity
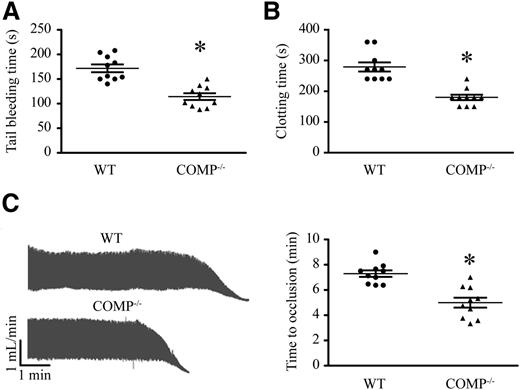
To investigate the effects of COMP on hemostasis, we first compared the tail bleeding time between COMP−/− and littermate C57/BL6 (WT) mice. Despite normal platelet counts and other hematologic parameters (supplemental Table 2), COMP−/− mice displayed significantly shortened tail-bleeding time compared with WT mice (Figure 1A; WT vs COMP−/−: 172 ± 25 seconds [median, 167 seconds; 95% confidence interval [CI], 154-189.6 seconds] vs 114 ± 22s (median, 111 seconds; 95% CI, 98.69-129.7 seconds], n = 10 for each group, P < .05). In addition, the clotting time was markedly reduced in COMP−/− mice compared with WT mice (Figure 1B; WT vs COMP−/−: 279 ± 47 seconds [median, 270 seconds; 95% CI, 245.4-312.6 seconds] vs 186 ± 28 seconds [median, 180 seconds; 95% CI, 166.3-205.7 seconds], P < .05), indicating that COMP may affect hemostasis. To further investigate the effects of COMP on thrombus formation in vivo, we performed a FeCl3-induced thrombosis assay using mouse carotid arteries. The time required for vessel occlusion was significantly reduced in COMP−/− mice compared with WT mice (Figure 1C; WT vs COMP−/−: 7.3 ± 0.8 min vs 5.0 ± 1.2 min, P < .05), indicating that COMP is involved in the thrombotic response.
Hemostasis and thrombosis in WT and COMP−/− mice. (A) Tail-bleeding time in WT and COMP−/− mice. n = 10, *P < .05. (B) Blood clotting time in WT and COMP−/− mice. n = 10, * P < .05. (C) Representative Doppler echocardiogram of the blood flow rate in mouse right carotid arteries following FeCl3 injury (left). The time when the flow rate was reduced to 0 mL/min was considered as the complete occlusion time. Statistic results of the right carotid artery complete occlusion time (right). n = 10, *P < .05.
Hemostasis and thrombosis in WT and COMP−/− mice. (A) Tail-bleeding time in WT and COMP−/− mice. n = 10, *P < .05. (B) Blood clotting time in WT and COMP−/− mice. n = 10, * P < .05. (C) Representative Doppler echocardiogram of the blood flow rate in mouse right carotid arteries following FeCl3 injury (left). The time when the flow rate was reduced to 0 mL/min was considered as the complete occlusion time. Statistic results of the right carotid artery complete occlusion time (right). n = 10, *P < .05.
COMP specifically targets thrombin activity
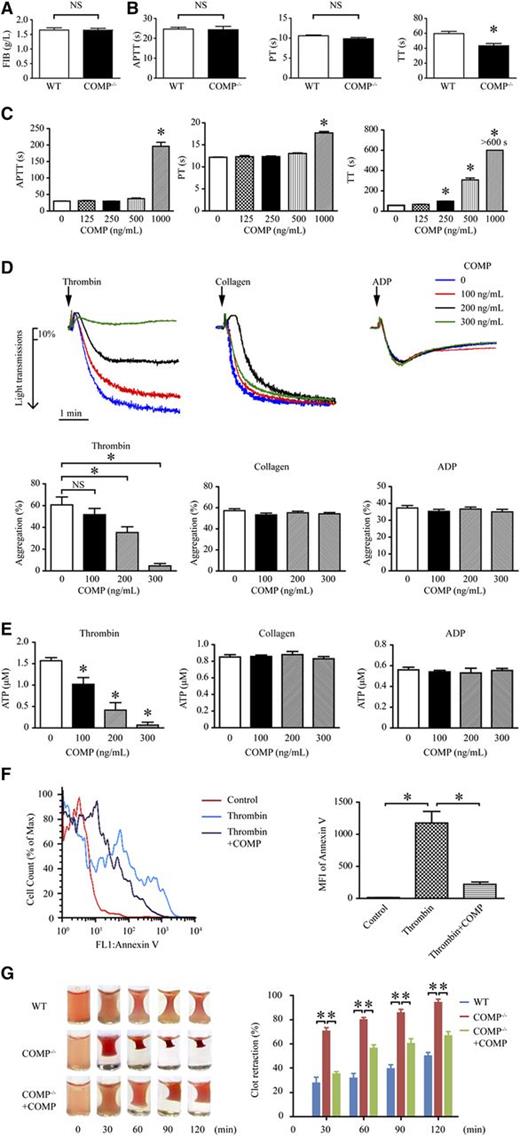
To further explore the COMP mechanisms affecting procoagulation, we compared fibrinogen values and coagulation parameters in platelet-free plasma between COMP−/− and WT mice. The fibrinogen concentration in plasma was comparable between WT and COMP−/− mice (Figure 2A; 1.65 ± 0.08 g/L vs 1.65 ± 0.06 g/L, n = 6). We further assessed the plasma coagulation activity using activated partial thromboplastin time (APTT), prothrombin time (PT), and thrombin time (TT) (Figure 2B). Although there was no significant differences in APTT (WT vs COMP−/−: 25 ± 0.9 seconds vs 24 ± 1.7 seconds) and PT (WT vs COMP−/−: 10.6 ± 0.2 seconds vs 9.9 ± 0.3 seconds), the TT value obtained from COMP−/− mice was greatly reduced compared with that obtained from WT mice (WT vs COMP−/−: 60 ± 2.9 seconds vs 43 ± 3.0 seconds, P < .05). Furthermore, exogenously purified COMP was further applied in APTT, PT, and TT experiments. Intriguingly, COMP dose-dependently (250-1000 ng/mL) prolonged the TT value in the plasma of WT mice, but only increased APTT and PT values at a high concentration (1000 ng/mL; Figure 2C). Similar observations were obtained in human plasma (supplemental Figure I). Together, these data indicate that COMP may affect thrombin activity during the cleavage of fibrinogen to fibrin.
COMP regulates thrombin activity. Serum fibrinogen (FIB, A), activated partial thromboplastin time (APTT), prothrombin time (PT), and thrombin time (TT) (B) of the platelet-free plasma isolated from WT and COMP−/− mice (n = 6, *P < .05; NS, not significant). (C) APTT, PT, and TT of WT platelet-free plasma in the absence or presence of purified COMP. Platelet-free plasma that had no coagulation at 600 seconds is shown as >600 seconds (n = 4, *P < .05 vs 0 ng/mL COMP). (D) Representative platelet aggregation tracings following thrombin (0.1 U/mL), collagen (1 μg/mL), or ADP (10 μM) treatment with or without purified COMP (top). Statistical results show the maximal percentage of platelet aggregation (n = 4, *P < .05 vs 0 ng/mL COMP) (bottom). (E) Measurement of ATP released in the supernatant of platelets treated with thrombin (0.1 U/mL), collagen (1 μg/mL), or ADP (10 μM) in the absence or presence of COMP for 5 minutes (n = 4, *P < .05 vs 0 ng/mL COMP). (F) Representative flow cytometry analysis of phosphatidylserine on the surface of platelets treated with thrombin in the presence or absence of COMP (300 ng/mL) for 15 minutes. Bar graph shows statistical results of the mean fluorescence intensity (MFI). n = 4, *P < .05. (G) Representative images of the clots in thrombin-induced mouse platelet-rich plasma with or without purified COMP (300 ng/mL) at different time points (left). Statistic bar graph shows the percentage of nonclot size to initial clot size (n = 4, *P < .05) (right).
COMP regulates thrombin activity. Serum fibrinogen (FIB, A), activated partial thromboplastin time (APTT), prothrombin time (PT), and thrombin time (TT) (B) of the platelet-free plasma isolated from WT and COMP−/− mice (n = 6, *P < .05; NS, not significant). (C) APTT, PT, and TT of WT platelet-free plasma in the absence or presence of purified COMP. Platelet-free plasma that had no coagulation at 600 seconds is shown as >600 seconds (n = 4, *P < .05 vs 0 ng/mL COMP). (D) Representative platelet aggregation tracings following thrombin (0.1 U/mL), collagen (1 μg/mL), or ADP (10 μM) treatment with or without purified COMP (top). Statistical results show the maximal percentage of platelet aggregation (n = 4, *P < .05 vs 0 ng/mL COMP) (bottom). (E) Measurement of ATP released in the supernatant of platelets treated with thrombin (0.1 U/mL), collagen (1 μg/mL), or ADP (10 μM) in the absence or presence of COMP for 5 minutes (n = 4, *P < .05 vs 0 ng/mL COMP). (F) Representative flow cytometry analysis of phosphatidylserine on the surface of platelets treated with thrombin in the presence or absence of COMP (300 ng/mL) for 15 minutes. Bar graph shows statistical results of the mean fluorescence intensity (MFI). n = 4, *P < .05. (G) Representative images of the clots in thrombin-induced mouse platelet-rich plasma with or without purified COMP (300 ng/mL) at different time points (left). Statistic bar graph shows the percentage of nonclot size to initial clot size (n = 4, *P < .05) (right).
Another important role of thrombin is to activate platelets.1 Exogenously purified COMP dose-dependently (100-300 ng/mL) inhibited thrombin (0.1 U/mL)-induced mouse platelet aggregation (Figure 2D). In contrast, COMP exhibited no effect on adenosine 5′-diphosphate (ADP) (10 μM)- or collagen (1 μg/mL)-induced platelet aggregation. The inhibitive effect of COMP on platelet aggregation was also validated in human washed platelets (supplemental Figure IIa). Concomitantly, COMP specifically prevented thrombin but not ADP or collagen-induced ATP release in mouse (Figure 2E) platelets. Similarly, COMP also negatively affected thrombin-induced ATP release in human platelets (supplemental Figure IIb). Moreover, the thrombin-induced translocation of phosphatidylserine from the inner to outer leaflet of the plasma membrane, as a marker of activated platelets detected by fluorescein-isothiocyanate–conjugated Annexin V, was repressed by purified COMP (300 ng/mL) (Figure 2F), indicating that COMP inhibited thrombin-induced platelet activation. In contrast, collagen-induced platelet activation was not affected by purified COMP (data not shown). In addition, the rate of thrombin-induced clot retraction in platelet-rich plasma from COMP−/− mice was markedly accelerated compared with that of WT mice, whereas exogenous supplementation with purified COMP (300 ng/mL) circumvented the enhanced clot retraction of COMP−/− platelet-rich plasma (Figure 2G). Thus, COMP may suppress the thrombin-regulated platelet retraction. Together, these findings suggest that COMP specifically targeted thrombin-induced platelet aggregation, secretion, activation, and retraction.
The pleiotropic effects of thrombin on cells are mediated by the protease activated receptor (PAR) family of G-protein–coupled receptors, which includes PAR-1 to PAR-4. Human platelets express PAR-1 and PAR-4, and the cleavage of each receptor initiates signaling cascades. Mouse platelets express PAR-3 and PAR-4, but PAR-3 does not mediate signaling, making PAR-4 the sole signaling receptor.25 To explore whether COMP affects thrombin-initiated platelet activation by affecting membrane-bound PAR receptors, the specific PAR-4 activator AYPGKF-NH2 was used to induce platelet aggregation. However, exogenous COMP at concentrations ranging from 100 to 300 ng/mL exhibited no effect on PAR-4 activator–induced platelet aggregation (supplemental Figure III). These data indicated that COMP may preferentially target thrombin rather than thrombin receptors. Thus, COMP may serve as endogenous modulator of thrombin.
SPR examination of the COMP-thrombin interaction
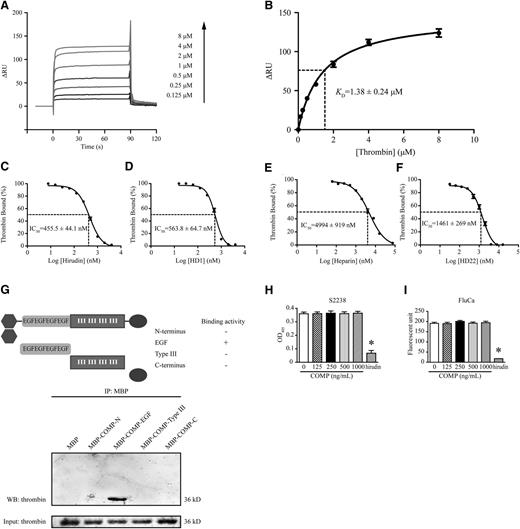
Next, we asked whether COMP directly binds to thrombin. An increasing amount (0.125-8 μM) of recombinant α-thrombin was injected over a CM5 sensor chip immobilized with purified COMP. A dose-dependent increase in surface plasmon resonance (SPR) signaling was observed after injection, indicating the binding of thrombin to COMP, although reduced signaling, demonstrating the dissociation process, was present after the injections ceased (Figure 3A). Notably, thrombin did not digest COMP within the duration of SPR experiments (supplemental Figure IV), indicating that the data obtained from SPR were from the direct binding of thrombin to full-length COMP rather than a COMP fragment. The 1:1 Langmuir model was applied for data stoichiometry best fitting to yield a KD value of 1.38 ± 0.24 μM for thrombin, implying COMP directly bound to thrombin with moderate affinity (Figure 3B, n = 3).
Direct interaction between COMP and thrombin. (A) Representative surface plasmon resonance analysis of different concentrations of thrombin injected through a biosensor chip conjugated with COMP. The altered response unit (ΔRU) is recorded in real time after perfusion. (B) The best fitting of plot data in thrombin concentrations and their corresponding ΔRUs yields a KD of 1.38 ± 0.24 μM for thrombin based on a 1:1 Langmuir model (n = 3). Thrombin (1 μM) in the presence of hirudin (C), HD1 (D), heparin (E), and HD22 (F) at different concentrations was injected over a COMP-linked biosensor chip. The percentage of ΔRU to the ΔRU in thrombin (1 μM) injection alone (thrombin bound) regressed according to the concentration of hirudin, HD1, heparin, or HD22 (n = 3). Concentrations corresponding to 50% of thrombin bound are the IC50. (G) Western blot of protein fractions precipitated with amylase beads from a mixture of thrombin (3 μg) and different purified MBP-fused COMP protein fragments. Fractions before precipitation were applied as input for loading control. Thrombin (0.1 U/mL)-cleaved chromogenic substrate S2238 (50 μM) (H) and fluorogenic substrate FluCa (2.5 mM) (I) were measured with absorbance at 405 nm and fluorescence intensity (excitation, 390 nm; emission, 460 nm), respectively. Hirudin (0.1 mg/mL) was applied as a positive control. n = 3, *P < .05 vs 0 ng/mL. IP, immunoprecipitation; OD405, optical density at 405 nm; WB, western blot.
Direct interaction between COMP and thrombin. (A) Representative surface plasmon resonance analysis of different concentrations of thrombin injected through a biosensor chip conjugated with COMP. The altered response unit (ΔRU) is recorded in real time after perfusion. (B) The best fitting of plot data in thrombin concentrations and their corresponding ΔRUs yields a KD of 1.38 ± 0.24 μM for thrombin based on a 1:1 Langmuir model (n = 3). Thrombin (1 μM) in the presence of hirudin (C), HD1 (D), heparin (E), and HD22 (F) at different concentrations was injected over a COMP-linked biosensor chip. The percentage of ΔRU to the ΔRU in thrombin (1 μM) injection alone (thrombin bound) regressed according to the concentration of hirudin, HD1, heparin, or HD22 (n = 3). Concentrations corresponding to 50% of thrombin bound are the IC50. (G) Western blot of protein fractions precipitated with amylase beads from a mixture of thrombin (3 μg) and different purified MBP-fused COMP protein fragments. Fractions before precipitation were applied as input for loading control. Thrombin (0.1 U/mL)-cleaved chromogenic substrate S2238 (50 μM) (H) and fluorogenic substrate FluCa (2.5 mM) (I) were measured with absorbance at 405 nm and fluorescence intensity (excitation, 390 nm; emission, 460 nm), respectively. Hirudin (0.1 mg/mL) was applied as a positive control. n = 3, *P < .05 vs 0 ng/mL. IP, immunoprecipitation; OD405, optical density at 405 nm; WB, western blot.
COMP binds to thrombin at exosites I and II through its EGF domain
Thrombin has 2 distinct electropositive surface domains, termed exosite I and exosite II, that contribute to the specificity of thrombin for recognizing its substrates, inhibitors, and receptors.26 To define the COMP mechanism for binding to thrombin, we used selective exosite ligands (ie, the DNA aptamer HD1 and hirudin to block exosite I and the DNA aptamer HD22 and heparin to block exosite II) for further SPR analysis. Both hirudin (Figure 3C) and HD1 (Figure 3D) competitively inhibited thrombin binding to immobilized COMP. The corresponding half maximal inhibitory concentration (IC50) values were 455.5 ± 44.1 nM for hirudin and 563.8 ± 64.7 nM for HD1. Similarly, heparin (Figure 3E) and HD22 (Figure 3F) inhibited thrombin binding to immobilized COMP (IC50 values: 4994 ± 919 nM for heparin and 1461 ± 269 nM for HD22). These finding suggested that COMP bound to thrombin via both exosite I and exosite II. To investigate the binding domain of COMP, we reconstructed maltose binding protein (MBP)-fused COMP domains, including the N terminus (amino acids: 20-83), epidermal growth factor (EGF)-like repeats (amino acids 84-261), type III repeats (amino acids 266-520), and the C terminus (amino acids 521-755). Only the MBP-fused EGF-like repeats of COMP pulled down purified thrombin, indicating direct binding of the COMP-EGF domain to thrombin (Figure 3G). However, COMP displayed no effects on the peptidase activity of thrombin despite the interaction with exosites I and II, demonstrated by the amidolysis of small peptide substrates S2238 and fluorogenic substrates (Figure 3H-I).
Bone marrow–derived COMP negatively regulates hemostasis and thrombosis
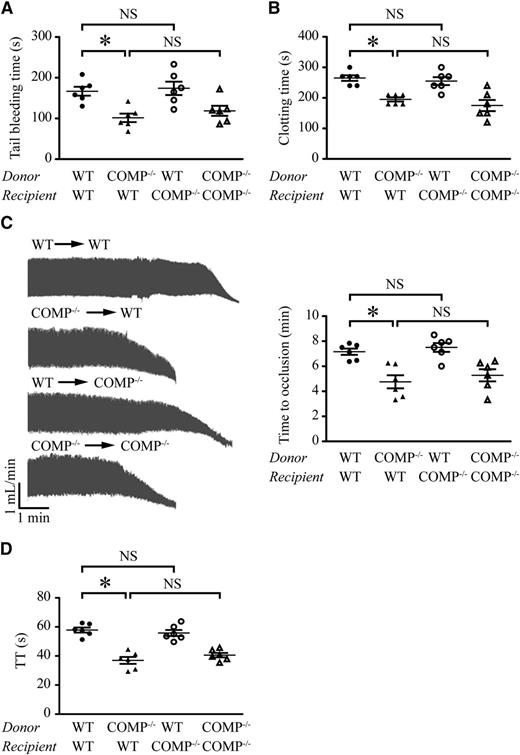
To evaluate the cellular origin of the COMP involved in hemostasis and thrombosis in vivo, we generated radiation chimera mice. Bone marrow cells were isolated from WT and COMP−/− mice and injected into lethally irradiated male WT and COMP−/− mice, respectively. To evaluate the degree of engraftment following irradiation, CD45.1+ cells in the peripheral blood were assessed by flow cytometry in CD45.2 mice transplanted with CD45.1 bone marrow cells. The percentage of CD45.1+ cells in peripheral leukocytes was not significantly different between WT and COMP−/− mice (70% vs 93%) following bone marrow reconstitution (data not shown). Polymerase chain reaction analysis of whole blood derived from chimeric mice further confirmed that the bone marrow from donor mice was reconstituted in recipient mice (supplemental Figure V). The hematologic values of the blood cells were comparable among the 4 groups of chimeric mice (supplemental Table 3). These results indicated that bone marrow reconstitution and refreshed blood profiles of the WT and COMP−/− recipient mice were equally modulated by irradiation-coupled transplantation. The tail bleeding time of WT mice engrafted with bone marrow from WT mice was 167 ± 27 seconds (median, 167 seconds; 95% CI, 138.5-195.2 seconds; n = 6), whereas COMP−/− bone marrow chimeras had markedly shortened bleeding time (102 ± 26 seconds [median, 100 seconds; 95% CI, 74.6-128.4 seconds; n = 6, P < .05]). In contrast, the bleeding time of COMP−/− mice engrafted with bone marrow from WT mice was similar to that of WT chimera mice (174 ± 40 seconds [median, 170 seconds; 95% CI, 132-216 seconds; n = 6]). There was no significant difference between WT and COMP−/− mice engrafted with bone marrow from COMP−/− mice (Figure 4A). Similar results were obtained regarding the clotting time (Figure 4B). Moreover, artery thrombosis was induced by FeCl3 in the 4 groups of the chimeric mice. In line with the above observations, COMP deficiency in the bone marrow but not in recipient mice accelerated FeCl3-induced thrombosis in vivo (Figure 4C). To explore whether the affected hemostasis and thrombosis in chimera mice was due to the altered thrombin activity in the presence or absence COMP, TT values were measured in chimeric mice after reconstitution (Figure 4D). Interestingly, COMP deficiency in the bone marrow of donor mice greatly shortened the TT time in both WT and COMP−/− recipient mice, whereas COMP deficiency in recipient mice did not significantly affect the TT compared with the respective WT recipients. These data provide direct evidence that the bone marrow–derived COMP is accountable for the inhibited thrombin activity and procoagulant function in vivo.
Bone marrow– or non–bone marrow–derived COMP in hemostasis and thrombosis. Chimeric mice were created by WT or COMP−/− (recipients) mice crosstransplanted with bone marrow from WT or COMP−/− mice (donors). (A-C) Tail-bleeding time (A), blood clotting time (B), and carotid artery occlusion time following FeCl3 injury (C) in chimeric mice. (D) TT was measured in the platelet-free plasma isolated from chimeric mice. n = 6 for each group. *P < .05; BM, bone marrow; NS, not significant.
Bone marrow– or non–bone marrow–derived COMP in hemostasis and thrombosis. Chimeric mice were created by WT or COMP−/− (recipients) mice crosstransplanted with bone marrow from WT or COMP−/− mice (donors). (A-C) Tail-bleeding time (A), blood clotting time (B), and carotid artery occlusion time following FeCl3 injury (C) in chimeric mice. (D) TT was measured in the platelet-free plasma isolated from chimeric mice. n = 6 for each group. *P < .05; BM, bone marrow; NS, not significant.
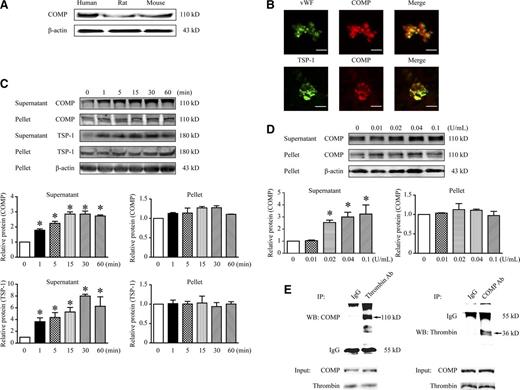
Platelets secrete COMP
Platelets are derived from megakaryocytes in the bone marrow; thus, we asked whether COMP is expressed in platelets. Indeed, COMP protein was detected by western blotting using human, rat, and mouse resting platelets (Figure 5A). Coimmunofluorescence staining further revealed the colocalization of COMP with von Willebrand factor and TSP-1, 2 recognized α-granule proteins, indicating the existence of COMP in resting platelets from humans (Figure 5B) and mice (supplemental Figure VI). Furthermore, COMP in thrombin-activated platelets was assessed. Considering the potential interference of divalent cations such as Ca2+ or Mg2+ on the platelet secretion of some glycoproteins such as TSP-1,27 we measured the COMP expression and secretion by activated platelets in the presence of EDTA. COMP was barely detectable in the supernatant of resting platelets. Notably, thrombin (0.1 U/mL) time-dependently increased COMP in the supernatant of platelets from 1 to 60 minutes (Figure 5C), in parallel with TSP-1 secretion, indicating COMP might be released from α-granules of platelets. Moreover, thrombin in the 0.02- to 0.1-U/mL concentration range dose-dependently stimulated COMP secretion (Figure 5D). In contrast, the COMP level in the cellular pellet of platelets was not significantly affected. Thus, the total COMP of platelets lysates containing both pellet and supernatant was increased following thrombin stimulation (supplemental Figure VIIa). Interestingly, application of cycloheximide, the inhibitor of protein biosynthesis, reversed the thrombin-elevated COMP in platelets. In contrast, the pretreatment of MG132, the inhibitor of proteasome degradation, had no effect on COMP upregulation (supplemental Figure VIIb), implying that thrombin-upregulated COMP expression within 1 hour was not related to inhibition of COMP degradation. Moreover, no significant change of COMP was detected on platelet surface following thrombin stimulation, excluding the possibility of thrombin-induced COMP membrane translocation (supplemental Figure VIIc). Together, these data indicated that the thrombin-enhanced COMP level was due to de novo protein synthesis in activated platelets. Moreover, other platelet activators including PAR-4 agonist, collagen, and ADP all significantly increased COMP expression in platelets lysates as well (supplemental Figure VIId). This finding implied that the increased COMP was mainly related to the platelet activation, which was further demonstrated when U73122, a phospholipase C inhibitor that inhibits platelet activation, blocked platelet-activation–induced COMP expression (supplemental Figure VIIe). To further confirm the bona fide interaction between thrombin and platelet-derived COMP, we performed coimmunoprecipitation assays using the supernatant of thrombin-stimulated platelets (Figure 5E). COMP was present in thrombin antibody precipitated samples but not in immunoglobulin G (IgG) controls. In addition, thrombin was only present in COMP antibody-precipitated samples but not in IgG controls. These data reinforce the ideas that activated platelets upregulate and secrete COMP and platelet-derived COMP is associated with thrombin.
COMP expression and secretion in platelets. (A) Western blot of suspensions of resting platelets without centrifugation from humans, rats, and mice. (B) Representative immunofluorescence images of human resting platelets (scale bar, 5 μm). Mouse washed platelets were treated with thrombin (0.1 U/mL) at different time points (C) or stimulated with thrombin for 60 minutes at different doses (D). Representative western blot analysis for supernatant and pellet of platelets isolated from platelet suspensions by centrifugation. Protein band density was normalized to the corresponding β-actin and then to the mean of the corresponding control group (0 minutes or 0 U/mL). Bar graphs show the band densitometry with statistics. n = 3, *P < .05 vs 0 minutes (C) or 0 U/mL (D). (E) Coimmunoprecipitation (IP) of COMP and thrombin in the supernatant of thrombin-activated mouse platelets (0.1 U/mL, 60 minutes). Rabbit IgG was as negative control for IP. Input fractions isolated prior to precipitation were detected for loading controls.
COMP expression and secretion in platelets. (A) Western blot of suspensions of resting platelets without centrifugation from humans, rats, and mice. (B) Representative immunofluorescence images of human resting platelets (scale bar, 5 μm). Mouse washed platelets were treated with thrombin (0.1 U/mL) at different time points (C) or stimulated with thrombin for 60 minutes at different doses (D). Representative western blot analysis for supernatant and pellet of platelets isolated from platelet suspensions by centrifugation. Protein band density was normalized to the corresponding β-actin and then to the mean of the corresponding control group (0 minutes or 0 U/mL). Bar graphs show the band densitometry with statistics. n = 3, *P < .05 vs 0 minutes (C) or 0 U/mL (D). (E) Coimmunoprecipitation (IP) of COMP and thrombin in the supernatant of thrombin-activated mouse platelets (0.1 U/mL, 60 minutes). Rabbit IgG was as negative control for IP. Input fractions isolated prior to precipitation were detected for loading controls.
Platelet-derived COMP inhibits thrombin activity and procoagulant function
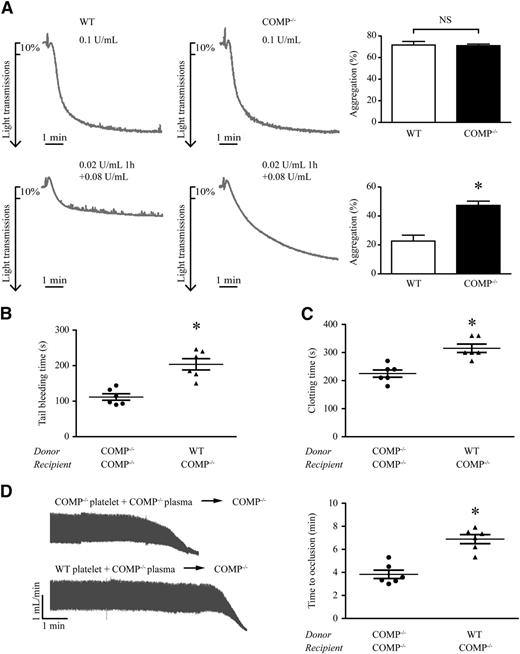
Next, we asked whether platelet-derived COMP regulates thrombin activity. To address this question, we first compared platelet aggregation in response to thrombin (0.1 U/mL) between WT and COMP−/− mice. Within 2 minutes, >70% of the platelets identically aggravated in both mouse groups (Figure 6A). However, pretreatment of the platelets with a low concentration (0.02 U/mL) of thrombin at 37°C for 1 hour, which did not cause platelet aggregation (data not shown) but significantly upregulated COMP expression and secretion (Figure 5D), led to the reduction of WT platelet aggregation by additional 0.08 U/mL thrombin stimulation compared with COMP−/− platelets (Figure 6A; WT vs COMP−/−: 23% ± 4.1% vs 47% ± 3.0%, P < .05). In contrast, pretreatment with thrombin (0.02 U/mL, 1 hour) in both WT and COMP−/− platelets led to the same decrease of PAR-4 agonist–induced platelet aggregation, implying no difference in PAR-4 receptor desensitization between 2 genotypic platelets (supplemental Figure VIIIa; WT vs COMP−/−: 64% ± 2.4%/44% ± 2.4% vs 62% ± 4.1%/45% ± 1.9% [without/with pretreatment]). Furthermore, thrombin-induced TSP-1 release displayed identically in WT and COMP−/− platelets (supplemental Figure VIIIb). These data indicate that platelet-derived COMP negatively regulates thrombin activity in vitro.
Platelet-derived COMP involvement in hemostasis and thrombosis. (A) Representative platelet aggregation tracings following different thrombin treatments: 0.1 U/mL (top) or 0.02 U/mL for 1 hour plus an additional 0.08 U/mL thrombin (bottom). Bar graph shows statistical results of aggregation maximal percentages from 4 independent results. *P < .05; NS, not significant. (B-D) Tail-bleeding time (B), blood clotting time (C), and carotid artery occlusion time (D) in COMP−/− mice with platelet transfusion. n = 6, *P < .05.
Platelet-derived COMP involvement in hemostasis and thrombosis. (A) Representative platelet aggregation tracings following different thrombin treatments: 0.1 U/mL (top) or 0.02 U/mL for 1 hour plus an additional 0.08 U/mL thrombin (bottom). Bar graph shows statistical results of aggregation maximal percentages from 4 independent results. *P < .05; NS, not significant. (B-D) Tail-bleeding time (B), blood clotting time (C), and carotid artery occlusion time (D) in COMP−/− mice with platelet transfusion. n = 6, *P < .05.
To further confirm the role of platelet-derived COMP on procoagulant function in vivo, washed platelets purified from WT and COMP−/− mice were reconstituted with COMP−/− platelet-free plasma, which was used to exclude the effects of COMP in WT plasma, and then transfused into recipient COMP−/− mice. COMP−/− recipient mice that received COMP−/− donor platelets had a tail-bleeding time of 112 ± 22 seconds (median, 106 seconds; 95% CI, 88.2-135.1 seconds, n = 6). Strikingly, the tail-bleeding time was greatly prolonged to 204 ± 39 seconds (median, 205 seconds; 95% CI, 162.9-244.5 seconds, n = 6) in COMP−/− recipient mice receiving WT donor platelets (Figure 6B). A similar finding was observed with regard to clotting time (Figure 6C). Moreover, WT platelet transfusion prolonged FeCl3-induced artery thrombus formation in COMP−/− mice compared with COMP−/− platelet transfusion (Figure 6D; 6.9 ± 1.0 minutes [median, 7.2 minutes; 95% CI, 5.9-7.9 minutes] vs 3.8 ± 0.9 minutes [median, 3.5 minutes; 95% CI, 2.9-4.8 minutes], n = 6). Taken together, these results highlight the importance of platelet-derived COMP as a critical negative-feedback regulator in thrombin-related hemostasis and thrombosis.
Discussion
Our study revealed COMP as a natural thrombin inhibitor and an endogenous anticoagulant protein. The upregulation and release of COMP from platelets upon activation may constitute an important negative-feedback regulation of thrombin-related blood coagulation.
The blood coagulation cascade cannot occur without the action of the serine protease thrombin. Therefore, direct inhibitors of thrombin are now being developed for a promising new generation of anticoagulants for the prevention and treatment of acute coronary artery diseases and thrombotic disorders, providing the advantages of efficacy, safety, and tolerability over heparins and vitamin K antagonists. In the search of direct inhibitors of thrombin, a number of compounds have been identified, including those derived from the tripeptide template D-Phe-Pro-Arg, aptamers, and peptides isolated from blood-sucking animals.28 Moreover, thrombin has nonhemostatic functions attributable to atherosclerosis and atrial fibrillation formation. For example, ApoE−/− mice with genetically reduced levels of thrombin or direct thrombin inhibitor have decreased atherosclerosis, increased plaque stability, and a decreased proinflammatory profile.29 The activity of thrombin is tightly regulated, but the regulatory mechanism is yet to be fully understood. Endogenous thrombin inhibitors include antithrombin, heparin cofactor II, protein C inhibitor, and protease nexin 1, which belong to the serpins family. A recent study has also indicated that β2-glycoprotein I functions as a physiological anticoagulant by inhibiting the activity of thrombin.30 Here, we identified COMP as a novel endogenous physiological regulator of thrombin. COMP directly binds to thrombin and specifically inhibits thrombin-induced platelet aggregation, activation, secretion, and contraction, as well as the conversion of fibrinogen to fibrin. Moreover, COMP−/− mice exhibited a shortened tail-bleeding time and accelerated thrombosis formation in injured vessels. Thus, by fine-tuning thrombin activity, COMP is not only important for maintaining hemostasis but also involved in pathological thrombosis.
Thrombin has 2 anion-binding exosites, exosites I and II, which are essential for its activity and specificity. Some thrombin ligands specifically bind to either exosite I or II, whereas others engage both exosites.26 Exosite I is the main binding site for fibrinogen, thrombomodulin, PAR1, factors V and VIII, protein C, and FXIII. Exosite II is known as a heparin-binding site and plays a role in platelet binding via GPIbα and the direct recognition of some substrates such as FV and FVIII. Here, we described COMP binding to thrombin at an approximate KD value of 1.38 μM, which is similar to the binding affinity of PAR-131 and fibrinogen32 to thrombin. In particular, the blockage of thrombin exosites with compounds specific for exosite I or II impaired the COMP-thrombin interaction, indicating that COMP binds to both exosites. However, COMP could not directly affect the peptidase activity of thrombin. Thus, this moderate COMP binding to thrombin may capture the binding sites for thrombin substrates and prevent excessive thrombin activation during physiological and pathological states.
Recent progress in the platelet transcriptome has revealed that thousands of genes are present in platelets.33 Resent advances in platelet proteomics have also uncovered hundreds of proteins released from activated platelets with unknown function, which may contribute to atherosclerotic or thrombotic diseases.34 Here, we identified COMP expression in α-granules from resting platelets released upon activation. Furthermore, bone marrow transplantation and platelet transfusion experiments indicated that platelet-derived COMP negatively regulated the coagulation cascade. Interestingly, TSP-1, one of the most abundant α-granule proteins belonging to the same TSP family as COMP, appears to exhibit a different function on hemostasis and thrombosis. TSP-1 is a trimeric glycoprotein secreted from α-granules from platelets upon their activation.7 TSP-1 potentially interacts with several platelet receptors including the integrins αvβ3 and αIIbβ3,35,36 CD36,37 and CD47.38 TSP-1 induces platelet activation likely by means of an indirect mechanism via CD36-dependent cyclic adenosine monophosphate/protein kinase A signaling pathway inhibition or by blocking the antithrombotic activity of nitric oxide/cyclic guanosine monophosphate signaling.39 In addition, TSP-1 protects endothelial von Willebrand factor from cleavage by ADAMTS13, enhancing the dynamic recruitment of platelets into developing thrombi. TSP-1−/− mice exhibit a prolonged occlusion time upon photochemical injury in venules and arterioles compared with WT vessels.40 In contrast, COMP is a pentameric glycoprotein that is released from platelets upon activation. COMP specifically inhibits thrombin activity by directly binding to thrombin. COMP does not affect ADP or collagen-induced platelet activation, nor does it interfere with the thrombin receptor. COMP−/− mice demonstrated shortened tail-bleeding and occlusion times upon vascular injury. These results demonstrate negative-feedback regulation of thrombin-induced platelet activation and the coagulation cascade by platelet-derived COMP. The importance of COMP in atherothrombosis and thromboembolic disease needs to be explored further. Targeting COMP-thrombin may shed light on the discovery of this novel anticoagulant.
The online version of this article contains a data supplement.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors are grateful for the technical support of Dr Weijuan Yao (PT, APTT, and TT, and the fibrinogen tests) and Prof Guoqing Liu (mice bone marrow transplantation) (Peking University). The support of Prof Chang Chen (Institution of Biophysics in Chinese Academy of Sciences) during SPR experiments is warmly appreciated. The authors thank Prof Oldberg Ake (Lund University, Sweden) for kindly providing the COMP−/− mice.
This research was supported by funding from the International Cooperation and Exchanges NSFC (81220108004) (W.K.), the National Program on Key Basic Research Projects (973 Program) (2012CB518002) (W.K.), the National Natural Science Foundation of the People’s Republic of China (81070243, 81121061, and 91339000) (W.K.), the National Science Fund for distinguished Young Scholars (81225002) (W.K.), the “111” Project of the Chinese Ministry of Education (B07001), and the National Science Fund for Young Scholars (81300198) (Y.F.).
Authorship
Contribution: Y.L. and Y.F. equally designed and performed the experiments, analyzed and interpreted the data and results, and wrote the manuscript; R.Q. performed platelet aggregation experiments; M.W., L.H., and F.Y. performed mouse blood collection and coagulation experiments; N.Y. organized and created the figures; J.Z. and C.-H.Y. provided suggestions for the experimental design; J.L. and X.W. designed portions of the study and edited the manuscript; and W.K. designed experiments, interpreted data, and wrote and edited the manuscript.
Correspondence: Wei Kong, Department of Physiology and Pathophysiology, Basic Medical College of Peking University, Beijing 100191, People’s Republic of China; e-mail: kongw@bjmu.edu.cn.
References
Author notes
Y.L. and Y.F. contributed equally to this study.