Abstract
Between 10% and 40% of newly diagnosed patients with acute myeloid leukemia (AML) do not achieve complete remission with intensive induction therapy and are therefore categorized as primary refractory or resistant. Few of these patients can be cured with conventional salvage therapy. They need to be evaluated regarding eligibility for allogeneic hematopoietic stem cell transplantation (HSCT) as this is currently the treatment with the highest probability of cure. To reduce the leukemia burden prior to transplantation, salvage chemotherapy regimens need to be employed. Whenever possible, refractory/relapsed patients should be enrolled in clinical trials as we do not have highly effective and standardized treatments for this situation. Novel therapies include tyrosine kinase inhibitors, small-molecule inhibitors (eg, for Polo-like kinase 1 and aminopeptidase), inhibitors of mutated isocitrate dehydrogenase (IDH) 1 and IDH2, antibody-based therapies, and cell-based therapies. Although the majority of these therapies are still under evaluation, they are likely to enter clinical practice rapidly as a bridge to transplant and/or in older, unfit patients who are not candidates for allogeneic HSCT. In this review, we describe our approach to refractory/early relapsed AML, and we discuss treatment options for patients with regard to different clinical conditions and molecular profiles.
Introduction
Primary refractory acute myeloid leukemia (AML) and early relapse remain among the most challenging scenarios in the management of AML. Primary refractory or resistant disease as defined by not achieving complete remission (CR) (ie, a remaining blast count of 5% or more after 1 to 2 cycles of intense induction therapy1 ) occurs in 10% to 40% of the patient population. Early relapse (mostly referred to by relapse within 6 months after CR1)2 must be differentiated from late relapse (>6 months) as response to salvage therapy, and overall survival (OS) is significantly different.3,4 Treatment decisions must be carefully taken and alternatives weighed against each other by looking at the complex picture of the patient and his or her age, performance status, comorbidities, cytogenetic findings, molecular profile, and patient preference.
The first question that we need to ask is whether there are means of reducing the risk for refractory disease and early relapse by the selection of the initial therapy; that is, is there a superior induction therapy compared with standard “7+3” (7 days of cytarabine and 3 days of anthracycline)? Although daunorubicin at 90 mg/m2 certainly is superior to 45 mg/m2 both in younger and the fit older patients5,6 including patients with FLT3-ITD,7 the direct comparison between daunorubicin 60 mg/m2 with 90 mg/m2 or 80 mg/m2 did not demonstrate any difference as to CR rate and OS8-10 including patients with FLT3-ITD,8 although higher-dose daunorubicin might improve outcome in core-binding factor AML.11 Thus, using 60 to 90 mg/m2 daunorubicin or 12 mg/m2 of idarubicin for 3 days is considered standard dosing. Whenever possible, we enroll newly diagnosed as well as relapsed patients into clinical trials. Outside trials, we use ICE (idarabucin 12 mg/m2 days 1, 3, and 5; cytarabine 100 mg/m2 continuously days 1-7; etoposide 100 mg/m2 days 1-3) as our standard induction therapy for fit patients tolerating intense treatment.12 In patients not fit for intensive chemotherapy, the induction therapy frequently has to be individualized taking patient and disease-specific characteristics into account.13 Outside trials, decitabine is our first choice for elderly patients unsuitable for intensive therapy. In countries where decitabine is not approved yet, low-dose cytarabine may be used instead. By introducing you to 2 patients from our practice, we would like to illustrate how we make treatment decisions for this patient group.
Patient 1
A 37-year-old woman was diagnosed with AML with 95% bone marrow blasts and a white count of 110 000/µL. Genetic analyses revealed a normal karyotype, an fms-like tyrosine kinase 3–internal tandem duplication (FLT3-ITD) with a length of 78 nucleotides and an allelic ratio of 0.67, and wild-type nucleophosmin 1 and CCAAT/enhancer-binding protein alpha (CEPBA). Induction chemotherapy with ICE was started, but the patient still showed 60% of blast in the bone marrow on day 15 indicating poor response. Therapy with FLA-IDA (fludarabine, cytarabine, idarubicine)14 was immediately started as second induction cycle and in addition as a bridge to allogeneic hematopoietic stem cell transplantation (HSCT). After this regimen, the patient achieved a partial remission with 8% bone marrow blasts. After 2 weeks without further chemotherapy, conditioning with fludarabine, amsacrine, cytarabine, and 4 Gy total body irradiation15 was initiated followed by allogeneic HSCT from her HLA-identical sister. Unfortunately, she relapsed 132 days after allogeneic HSCT. In the relapse sample, FLT3-ITD could be detected with an increase in the allelic ratio from 0.67 at time of diagnosis to 3.4 at time of relapse. After reduction of immunosuppressive therapy, the patient was started on a regimen consisting of sorafenib and 5-azacytidine (AZA) based on phase 2 data.16 With this regimen and 2 additional donor lymphocyte infusions, the patient’s AML was moderately controlled for 2 months. The aim was a second allogeneic HSCT with an alternative donor, but unfortunately she died because of septicemia related to her underlying AML.
Discussion for patient 1
Before diagnosing primary refractory disease, one has to define the time point when this has been determined. Primary refractory disease is usually diagnosed in patients who have not achieved CR after 2 cycles after induction chemotherapy.1 Beyond this standard definition, it has consistently been shown that the prognosis is dismal in patients who are refractory to the first induction cycle as indicated by persistence of a substantial amount of bone marrow blasts at day 15 or who do not achieve at least a partial remission at day 21 to 35.17-19 Taking this into account, response-adapted induction therapy with dose intensification during the second induction cycle is frequently12,19,20 implemented in cases of insufficient response to first induction therapy as in our patient. In patient 1, the early assessment at day 15, which we routinely do in our practice, opened the possibility to intensify treatment in a timely manner. Based on our recent analysis intensification using either high-dose cytarabine in combination with gemtuzumab ozogamicin and all-trans retinoic acid or a regimen based on high-dose cytarabine plus fludarabine significantly improved the CR rate.21 The latter is in line with the report from the Medical Research Council showing an earlier achievement of CR with FLAG-IDA (fludarabine, high-dose cytarabine, idarubicin, granulocyte colony-stimulating factor [G-CSF]) compared with standard 7+3 induction therapy.22 Our patient responded rather well to FLA-IDA with 8% residual blasts and proceeded directly to an allogeneic HSCT. However, if the marrow had been hypoplastic with 5% to 10% blast cells, we would have repeated the induction cycle with the identical chemotherapy as soon as clinically feasible (ie, in the absence of uncontrolled infection), as these patients do as well with conventional chemotherapy as with high-dose cytarabine based regimens.23 Once these patients have achieved CR after the second induction cycle, they have the same long-term outcome as patients who achieve complete blast clearance with the first induction cycle.14 The question of whether a second FLA-IDA before an allogeneic HSCT would have been beneficial cannot be answered based on currently available data. However, data from matched related donor (MRD) assessment before allogeneic HSCT indicating that a lower leukemia burden with a negative MRD assessment before allogeneic HSCT is associated with a better outcome after transplant24,25 might be in favor of a second cycle. After early relapse, our patient received sorafenib, a multi–tyrosine kinase inhibitor (TKI) in relapse after allogeneic HSCT. The use of a sorafenib in this situation holds some promise, as sorafenib may synergize with allogeneic immune effects to induce remissions.26 Furthermore, there appear to be synergistic effects between sorafenib and AZA.16
Patient 2
A 58-year-old female who was treated with standard intense therapy (ICE) in our institution for core-binding factor AML with inversion 16 (inv16) (c-kit receptor tyrosine kinase wild type) in an interventional treatment trial. She achieved CR after 1 cycle of induction therapy but relapsed at 3 months after completing her consolidation therapy with 3 cycles of high-dose cytarabine. At the time of relapse the patient was pancytopenic with an absolute neutrophil count of 100/µL. An echocardiogram revealed a reduced cardiac ejection fraction of 44%. Based on high second CR rates in patients with inv(16)-AML even in the situation of an early relapse, we recommended an intensive salvage therapy in this situation. Because of her reduced ejection fraction, the patient received a modified FLA-IDA regimen with liposomal daunorubicin replacing idarubicin. During therapy, the patient became febrile and diagnostic workup showed multiple nodules in a computed tomography scan consistent with aspergillosis of the lung. The patient was treated with antifungal therapy (voriconazole), but her respiratory situation did not improve and she needed mechanical ventilation. Granulocyte transfusions were given to the patient, and she slowly improved and could be extubated. Her neutrophil count normalized on day 32. Bone marrow biopsy revealed a CR morphologically. The patient went on to allogeneic HSCT from a fully HLA-compatible unrelated donor using reduced intensity conditioning according to the fludarabine, amsacrine, cytarabine, and 4 Gy total body irradiation protocol.15 Three years later, the patient is alive in CR.
Discussion for patient 2
This patient showed 2 clinically relevant problems in the setting of her relapsed disease: cardiac insufficiency and uncontrolled infection. Cardiac insufficiency is especially relevant when anthracyclines are considered. Many patients with relapsed AML have already received high cumulative doses of anthracycline. In this situation, replacing conventional anthracycline by liposomal daunorubicin should be considered as it does not reduce its efficacy27,28 while being less cardiotoxic as shown in pediatric AML protocols. Because prolonged neutropenia is a common problem in relapsed/refractory patients, fungal and bacterial infections present a major problem. For some patients not responding to broad-spectrum antibiotic or antifungal therapy, granulocyte infusions can be helpful as a bridge to regeneration.29 Interestingly, patients with inv16 have a higher likelihood of responding to salvage therapy compared with patients in other cytogenetic groups including patients with t(8;21).30
If standard therapy fails
Looking at the scenarios, it becomes evident that there is not 1 standard therapy for all patients with refractory/early relapse AML. Is there a way to predict the probability of resistant disease at the time of AML diagnosis? Walter et al tried to answer this question based on data from 4601 newly diagnosed AML patients who were treated with standard induction therapy within the National Cancer Research Institute (NCRI), Hemato-Oncologie voor Volwassenen Nederland (HOVON), Schweizerische Arbeitsgemeinschaft für Klinische Krebsforschung (SAKK), Southwest Oncology Group (SWOG), or MD Anderson Cancer Center AML study groups. Prognostic factors for failure to achieve CR included age, performance status, white blood cell count, secondary disease, cytogenetic risk group, and FLT3-ITD/nucleophosmin 1 (NPM1) mutation status. However, the area under the receiver operator characteristic curves was only 0.78 meaning that it was difficult to forecast primary resistance based on these parameters.31 Nonetheless, there are clear associations of distinct genotypes and a high probability of resistant disease to induction therapy, for example, AML characterized by a monosomal karyotype, an inv(3)/t(3;3) or a p53-alteration.32 Not only the likelihood of refractory/early relapse AML but also the prognosis for refractory/early relapsed patients is dependent on cytogenetic and molecular features. Several scoring systems have been introduced for patients with refractory/relapsed AML in order to identify patients with an improved outcome including the one from the HOVON and the Groupe Ouest-Est d'Etude des Leucémies Aigues et autres Maladies du Sang (GOELAMS) study group (Tables 1 and 2).33 ,34,35 Cytogenetics other than favorable, mutated FLT3 (FLT3-ITD), higher age, previous HSCT, and a short duration of first CR in relapsed patients were all adverse risk factors. Again, our patients had different adverse risk factors. Although patient 1 was young, her molecular profile revealed a FLT3-ITD as a risk factor. In patient 2, the inv16 was a favorable factor in the relapse situation. Similarly, relapsed patients with the genotype double-mutated CEBPA also have a high likelihood of a second CR and a favorable long-term survival after allogeneic SCT after relapse.36 When we diagnose primary refractory/early relapse AML, we decide primarily whether the patient is a candidate for allogeneic HSCT.12 At this point, allogeneic HSCT from a matched related or unrelated donor is the treatment strategy with the highest probability of cure,37,38 although survival does not exceed 20% to 35% after 4 years.12,39 If not already done at the time of primary diagnosis, we initiate a rapid donor search. Risk factors prompting us to look for suitable donors already at the time of diagnosis include unfavorable cytogenetics based on European Leukemia Net recommendations2 and molecular markers including FLT3-ITD (especially if allelic ratios of >0.51).32,40,41 For us, there is no clear-cut age limit for allogeneic HSCT. The Hematopoietic Cell Transplant–Comorbidity Index introduced by Sorror et al in 2005 (and adjusted for age in 2014) gives a good guidance for comorbidity-associated risk assessment and should be calculated for all patients when considering transplantation.42,43
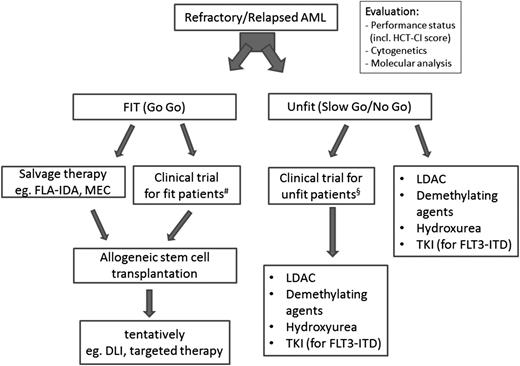
If the patient is not a candidate for allogeneic HSCT (eg, because of comorbidities or patient choice), we look at other treatment modalities including new drugs and/or palliative therapy with the aim to prolong the patient’s life with a meaningful quality of life (Figure 1). Outside trials, we start with low-dose cytarabine mainly to control leukocyte counts combined with best supportive care (blood transfusions, antibiotic/antifungal treatment). In some of our frailest patients, hydroxurea or 6-mercaptopurine to control hyperleukocytosis is currently the only treatment option. Palliative care services should be integrated in patients’ care. Calculations for the gain in quality-adjusted life-years for different treatment options do not exist in Germany and are therefore not considered in our deliberations for treatment allocation.
Algorithm for patients with early relapsed/refractory AML. When diagnosing primary refractory AML/early relapse patients need to be evaluated for allogeneic HSCT and/or a suitable trial. DLI, donor lymphocyte infusion; HCT-CI, Hematopoietic Cell Transplant–Comorbidity Index; LDAC, low-dose cytarabine; MEC, mitoxantrone, etoposide, cytarabine. #, § indicate that clinical trials should consider the patient’s performance status; that is, fit patients should receive more intense study medications (in most cases including chemotherapy) compared with unfit patients.
Algorithm for patients with early relapsed/refractory AML. When diagnosing primary refractory AML/early relapse patients need to be evaluated for allogeneic HSCT and/or a suitable trial. DLI, donor lymphocyte infusion; HCT-CI, Hematopoietic Cell Transplant–Comorbidity Index; LDAC, low-dose cytarabine; MEC, mitoxantrone, etoposide, cytarabine. #, § indicate that clinical trials should consider the patient’s performance status; that is, fit patients should receive more intense study medications (in most cases including chemotherapy) compared with unfit patients.
Chemotherapy before allogeneic HSCT
If allogeneic HSCT is considered as best treatment option for the patient, we need to select the most promising salvage regimen to induce remission of the disease as a “bridge to transplantation.” The aim of the salvage therapy is to reduce the leukemic burden, as one of the most significant factors for all survival end points after allogeneic HSCT is the disease status before allogeneic HSCT. The lower the leukemia burden prior to transplantation the better the outcome.12,44 Although salvage chemotherapy leads to CR rates of 40% to 60%, if CR duration was 1 year or longer, this rate drops to 10% to 15% in cases of shorter CR duration37 with the exception of AML with inv(16) or double-mutated CEBPA.30,36 Are there any superior salvage/second-line chemotherapies? Salvage therapy often includes additional drugs that have not already been used during the first induction cycle. Although a number of trials have examined different combination salvage therapies (Table 3),45-67 there is still no commonly accepted standard in this situation. Whenever possible, we include patients in a clinical trial for relapsed/refractory AML patients. Outside trials, we use FLA-IDA as a salvage regimen.20,45,68,69 If there are no infectious complications we do not add G-CSF to this regimen as there is no data supporting its use. However, idarubicin as an anthracycline appears to be an important drug in this combination as FLA alone (fludarabine, high-dose cytosine) was inferior to cytosine arabinoside, daunorubicin, and etoposide as reinduction in a study for relapsed/refractory patients.70 CR rates can be expected with this regimen in the range of 30% to 50%.68 Other regimens are comparable in their effectiveness in this situation. For instance, daunorubicin has been replaced by mitoxantrone as an alternative anthracycline in combination with cytarabine and etoposide (MEC).46 High-dose cytarabine in combination with mitoxantrone is also a common regimen in refractory/relapsed AML.47 Adding other purine analogs to salvage chemotherapy is also practiced in this situation, but these drugs can increase toxicity. The combination of clofarabine with high-dose cytarabine improved the response rate compared with high dose cytarabine alone but not OS.48 Cladribine is also an important purine analog used in the treatment of relapsed childhood AML,71 although not fulfilling its promise in relapsed adult AML.72 Sapacitabine is an oral purine analog that was investigated in elderly AML patients with newly diagnosed AML or relapsed AML showing some efficacy.73 When elacytarabine was compared with 7 other commonly used AML salvage therapies according to investigator´s choice, outcome was not significantly different between patients receiving elacytarabine or treatment in the control arm.49 Other modalities of reducing treatment toxicity is liposomal delivery of chemotherapy. CPX-351, a liposomal formulation of cytarabine:daunorubicin, has shown good efficacy in treatment related AML.74 A recent phase 2 study evaluated CPX-351 vs intense salvage therapy in relapsed patients. Here, a benefit for CPX-351 was seen for relapsed patients with a poor-risk profile34 (see Tables 1 and 2) with regard to response rates (39.3% vs 27.6%), EFS, and OS.50
Donor selection for allogeneic HSCT
Whenever possible, we aim for allogeneic transplantation for patients in second CR because there are essentially no cures with chemotherapy alone. However, if patients do not achieve a significant cytoreduction in the bone marrow or have more than 25% blasts in bone marrow, we usually abstain from transplantation and recommend hypomethylating agents or trial participation (and reevaluation after these therapies). The source of the graft (peripheral blood stem cells vs bone marrow) as well as MRD or matched unrelated donor do not impact significantly on outcome.12,75 If no HLA-matched donor is available, alternative graft sources including cord blood or stem cells from a haploidentical donor should be considered in these high risk patients. However, in a head to head comparison results had been inferior with cord blood or haploidentical donors compared with MRD and matched unrelated donor.12 For patients in second CR, we tolerate less well-matched donors because there are essentially no cures with chemotherapy alone in second CR.
Novel targets
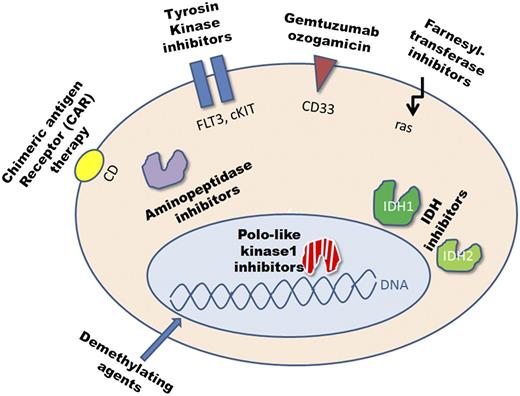
It is unlikely that patients with refractory AML will be cured solely by changing and improving current chemotherapy regimens. The candidate targets for novel therapeutic approaches in AML are diverse (Figure 2). Some of these novel approaches have already been studied even in phase 3 trials, others are just entering the early clinical trial phase or are under development. Although outside clinical trials the majority of these drugs are currently not available, we would still like to discuss these drugs because relapsed/refractory patients can be included in trials with these drugs and some of them are likely to be licensed soon. The list of targets and approaches is long and they can be classified in the following groups: epigenetic modifiers (demethylating agents, histone deacetylase inhibitors), antibody-based therapies (eg, GO), TKIs (eg, TKI against FLT3-ITD), small-molecule inhibitors of kinases involved in cell division (eg, Polo-like kinase 1), inhibitors of mutated enzymes (eg, inhibitors for IDH1, IDH2), aminopeptidase inhibitors (eg, Tosedostat), DOT1-like inhibitors (eg, EPZ-5676) for MLL-rearranged (MLL-r) leukemia, cell-based therapies including chimeric antigen receptor therapy, and immunomodulating agents.
The candidate targets in AML. cKIT, c-kit receptor tyrosine kinase; IDH, isocitrate dehydrogenase; ras, rat sarcoma.
The candidate targets in AML. cKIT, c-kit receptor tyrosine kinase; IDH, isocitrate dehydrogenase; ras, rat sarcoma.
The individual approach for each patient depends on several factors: the patient’s molecular profile (eg, mutations in IDH1/IDH2, FLT3-ITD, MLL-r), trial availability, the patient’s performance status, and so forth.
Targeting FLT3
FLT3 is an important target for patients with an activating FLT3 mutation. Although 30% of younger AML patients show a FLT3-ITD at the time of diagnosis, the percentage of patients rises in the population of patients with relapsed/refractory disease, as FLT3-ITD is highly associated with refractory/relapsed disease especially with a high allelic ratio. Several TKIs (eg, sorafenib, midostaurin, quizartinib, crenolanib) have been introduced to the treatment of these patients. It is important to keep in mind that these TKIs vary significantly in their specificity and their activity against resistance-conferring kinase domain mutations in FLT3.76 This may translate to differences in their clinical efficacy. Thus, it is unlikely that the question of the efficacy of TKIs will be solved in the very near future. FLT3 inhibitors as monotherapy have only led to transient responses.77 For some TKIs results from a phase 3 trial are available, whereas for others phase 1 and 2 trials are just being initiated. Should our 37-year-old patient in case 1 have received targeted therapy with a TKI directed against FLT3 at the time of induction therapy or soon after? The answer is complex and cannot be definitely given at this point. In a randomized placebo controlled phase 3 trial, no benefit was shown for elderly newly diagnosed AML patients treated with sorafenib in combination with intense therapy compared with patients receiving placebo.78 Importantly, there was also no outcome benefit seen in the very small subgroup of patients with FLT3-ITD treated with sorafenib, and patients in the sorafenib arm showed a higher treatment-related mortality.78 However, a recently presented study in newly diagnosed younger AML patients showed a benefit for EFS and relapse-free survival if sorafenib was added to standard therapy. This effect was independent of FLT3 mutational status.79 Other TKIs against FLT3 include crenolanib, quizartinib (AC220), lestaurtinib, PLX 3397, ASP 2215, and so forth. They are currently being studied and hold promise because of the high selectivity for FLT3. Quizartinib has already been shown to be effective in patients with relapsed or refractory AML with FLT3-ITD and also some patients with wild-type FLT3.80 Lestaurtinib was evaluated against placebo in relapsed FLT3-ITD positive patients after receiving chemotherapy. Here, no benefit in survival or response was observed in the lestaurtinib treated patients.81 The expectations toward efficacy of a TKI against FLT3 might be scaled down by the fact that FLT3-ITD is likely not an early mutation in clonal evolution82 and thus less promising as a target.
Targeting IDH1/IDH2
Since the discovery of IDH1 and IDH2 mutations in ∼10% of AML patients,83-87 inhibitors of IDH1 and IDH2 are already being introduced to clinical trials. Mutant IDH1 and IDH2 appear to be ideal pharmacologic targets as enzymes can be more easily targeted compared with other mutated structures in AML. AG-221, an oral IDH2 inhibitor, is the first of its kind being already studied in a phase 1 dose escalation clinical trial. Here, AG-221 was studied as a monotherapy in IDH2 mutated patients and the clinical response rate was promising in the first interim analysis.88 The introduction of IDH1 inhibitors will follow.
Targeting MLL
Targeting CD33
GO presents a combination of calicheamicin and a recombinant humanized IgG4 antibody directed against CD33. Several phase 1/2 trials have looked at its utility in primary refractory/relapsed disease but larger phase 3 trials in this setting are missing. CR rates between 32% and 55% were observed for combinations of GO with chemotherapy.91-93 Interestingly, data from the MRC 15 trial showed that the addition of GO to induction therapy might be of significant benefit for patients with CBF AML.94 Although GO is not easily available outside clinical trials, we try to get this drug based on published evidence in elderly relapsed patients with CD33 expression of the blasts as a bridge to transplantation.95 GO has also been used in combination with demethylating agents such as vorinostat96 or AZA.97 These preliminary studies are especially promising for patients not tolerating intense chemotherapy, and modifications of the antibody and its linker might lead to further improvement. For patients who receive GO with the goal of allogeneic HSCT especially sinusoidal obstructive syndrome (SOS) of the liver is a concern.98 No clear risk factors for the occurrence of SOS during SCT could be identified, but the rate of death related to SOS was found to be small.98
Other targets
The quinolone derivative vosaroxin inhibits the topoisomerase II and acts independently of the p53 mutational status. This drug was recently evaluated in combination with cytarabine (1 g/m2 on days 1-5) in a phase 3 study in patients with refractory or relapsed AML. A benefit in OS of 1.4 months (7.5 months vs 6.1 months) was observed in the overall cohort that just missed significance.99 Inhibitors of cell division such as the Polo-like kinase 1 inhibitor volasertib showed promise for AML patients.100 The oral aminopeptidase inhibitor tosedostat was studied in a multicenter phase 2 trial (NCT00780598) in elderly patients with refractory/relapsed AML with an overall response rate of 22%.
Immunologic approaches
Immune based approaches including chimeric antigen receptor therapies are still very immature and the immense operating expenses do not allow a general approach at this point. However, already well known immunomodulating agents such as lenalidomide might play a role in future AML therapy, especially in low proliferative disease.101
Finally, epigenetic modifiers could help patients with relapsed/refractory patients. Their role might be increased by combination therapy with novel agents (tosedostat, midostaurin) or as a bridge to allogeneic transplantation for less fit patients with the perspective of receiving reduced intensity conditioning. This is based on the assumption that these drugs sensitize tumor cells to cytotoxic agents by reexpression of epigenetically silenced tumor suppressor genes.102
In summary, the landscape of novel agents is very exciting and diverse. Although some agents might only be applicable for molecularly defined subgroups of AML patients (eg, IDH1/2 mutations, FLT3-ITD, MLL-r), other agents hold promise for a broad unselected group of relapsed/refractory AML patients.
Perspective
The prognosis of refractory/early relapse AML patients remains poor even with allogeneic SCT. Our insight into the molecular landscape of AML has dramatically increased with the introduction of next-generation sequencing as it has allowed us to identify novel genetic alterations including recurrent driver mutations. Functional analysis of these genetic aberrations has helped us to unravel the process of leukemogenesis further. For patients with refractory/early relapsed AML treatments arising out of these efforts present the most promising approaches.
Acknowledgments
This study was supported by grants from Deutsche Krebshilfe (110284, 110287, 110292 and 111267); the Deutsche José Carreras Leukämie-Stiftunge.V (grant DJCLS R13/14), the German Federal Ministry of Education and Research (grant 01EO0802) (IFB-Tx), Deutsche Forschungsgemeinschaft (grants HE 5240/5-1 and HE 5240/6-1), and Dieter-Schlag Stiftung.
Authorship
Contribution: F.T., R.F.S., M.H., and A.G. wrote the manuscript; and all authors read and agreed to the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Arnold Ganser, Department of Hematology, Hemostasis, Oncology, and Stem Cell Transplantation, Hannover Medical School, Carl-Neuberg Str 1, 30625 Hannover, Germany; e-mail: ganser.arnold@mh-hannover.de.