Abstract
Introduction: Chronic lymphocytic leukemia (CLL) cells typically carry one or more clonally rearranged immunoglobulin (Ig) genes. The specific sequence of an Ig rearrangement, or clonotype, can serve as a marker of minimal residual disease (MRD) in blood or bone marrow samples. The clonoSEQ assay (Adaptive Biotechnologies Corp) employs a next-generation sequencing (NGS) based MRD quantification method that uses consensus primers to amplify and then sequence the entire repertoire of Ig genes in a clinical sample. Each Ig clonotype, including the one marking a patient's CLL, can then be individually quantified with high precision. We previously demonstrated the utility of this NGS approach for predicting relapse-free survival in a single-institution cohort of CLL patients undergoing reduced-intensity allogeneic hematopoietic cell transplantation (RIC alloHCT). In this landmark analysis, we now assess the prognostic utility of molecular MRD quantification one year following RIC alloHCT in a multi-institution cohort.
Methods: We retrospectively evaluated the outcomes and MRD status of patients surviving without relapse to one year after RIC alloHCT for CLL. Patients were assessable for MRD based on availability of a tumor specimen from which to determine the CLL-associated Ig heavy chain (IgH) clonotypes and a cryopreserved peripheral blood mononuclear cell (PBMC) aliquot obtained one year post-HCT. Forty-six patients underwent conditioning with total lymphoid irradiation and anti-thymocyte globulin (TLI/ATG) and 56 were conditioned with fludarabine and busulfan (Flu/Bu) regimens. Clonal IgH rearrangements were identified by NGS-based IgH profiling in CLL-bearing blood or marrow samples obtained prior to HCT and these clonotypes were quantified in the IgH repertoire of PBMC samples from one year (+/- 90 days) post-HCT.
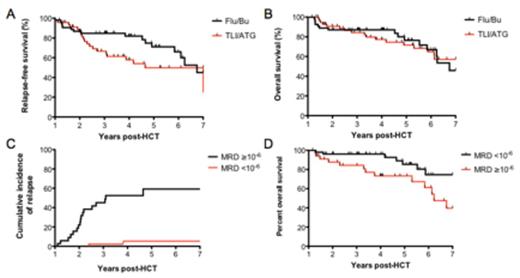
Results: One hundred two patients were assessable for outcomes beyond the one year post-HCT landmark. Amongst 97 patients with a diagnostic sample available, the IgH clonotype calibration rate was 90%. Five archived diagnostic samples yielded insufficient DNA to proceed with clonotype identification. 87/102 (85%) patients had paired tumor and one year PBMC samples suitable for MRD quantification. Patients receiving TLI/ATG or Flu/Bu conditioning had similar clinical characteristics. Median relapse-free survival (RFS) from the one year landmark was 4.7 years with TLI/ATG and 6.8 years with Flu/Bu (p=0.08; Figure 1A), and overall survival was not significantly different (Figure 1B). Acute GVHD (grades 2-4) occurred in 15.6% with TLI/ATG and 37.5% with Flu/Bu. Chronic GVHD occurred in 53% and 77%, respectively, with less extensive grade chronic GVHD using TLI/ATG (39%) compared with Flu/Bu (73%). Amongst patients alive and in remission one year after RIC alloHCT, peripheral blood MRD <10-6 was associated with more favorable outcomes, with just 5.3% relapsing within 7 years post transplant, compared with 59% relapse in those with ≥10-6 MRD (HR 11, 95% CI 4.3-28, p<0.0001; Figure 1C). MRD negativity one year post-HCT was also associated with improved long term overall survival (HR 2.6, 95% CI 1.1-6.4, p=0.03; Figure 1D).
Conclusions: In this non-randomized retrospective analysis of outcomes after RIC alloHCT for high-risk CLL, use of TLI/ATG or Flu/Bu conditioning regimens were associated with similar RFS, though a trend toward earlier relapse with TLI/ATG is observed. Flu/Bu was associated with substantially higher rates of acute and chronic GVHD and higher rates of death in remission. Irrespective of conditioning regimen used, molecular MRD quantification at one year post-HCT provides strong positive and negative predictive value for subsequent relapse. Identification of MRD positive patients at risk for relapse may permit the implementation of post-HCT immune modulation or maintenance treatment to reduce relapse risk for those in need, but spare MRD negative patients from the potential for additional toxicity.
Relapse-free (A) and overall (B) survival in high-risk CLL patients undergoing RIC alloHCT following TLI/ATG or Flu/Bu conditioning. At one year after RIC alloHCT, peripheral blood MRD ≥10-6 strongly predicts increased risk of relapse (p<0.0001) (C) and decreased overall survival (p=0.03) (D).
Relapse-free (A) and overall (B) survival in high-risk CLL patients undergoing RIC alloHCT following TLI/ATG or Flu/Bu conditioning. At one year after RIC alloHCT, peripheral blood MRD ≥10-6 strongly predicts increased risk of relapse (p<0.0001) (C) and decreased overall survival (p=0.03) (D).
Logan:Pharmacyclics: Consultancy; Amgen: Consultancy; Jazz Pharmaceuticals: Consultancy. Herrera:Genentech: Research Funding; Sequenta, Inc.: Research Funding; Pharmacyclics: Research Funding. Rezvani:Pharmacyclics: Research Funding. Kong:Adaptive Biotechnologies Corp: Employment, Equity Ownership. Faham:Adaptive Biotechnologies Corp.: Employment, Other: Stockholder. Miklos:Pharmacyclics: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract