In this issue of Blood, a new study by Kiyasu et al used a double-staining technique to identify cell-specific programmed cell death ligand 1 (PD-L1) expression in 1253 diffuse large B-cell lymphoma (DLBCL) samples and analyzed the findings together with programmed death-1 (PD-1) expression in tumor-infiltrating lymphocytes (TILs) and clinical outcomes, providing new insights into the role of the PD-1/PD-L1 pathway in DLBCL.1
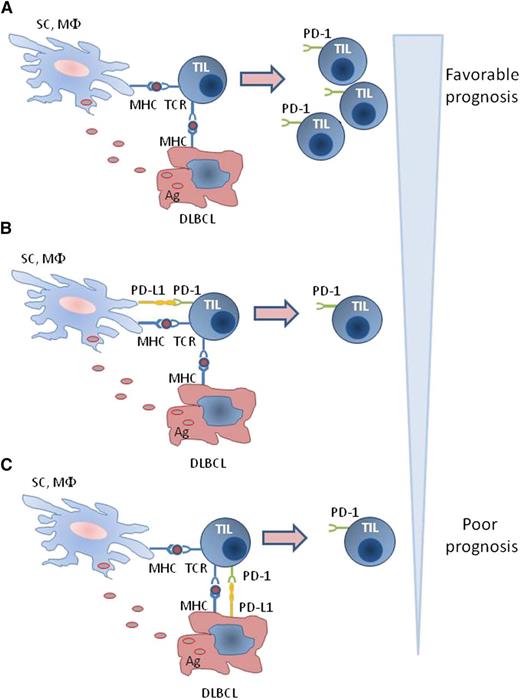
Regulation of PD-1 expression in T cells of the DLBCL microenvironment. (A) Direct and indirect antigen presentation by lymphoma cells and nonmalignant cells, respectively, in the absence of PD-L1 results in activation of TILs inducing expression of activation markers including PD-1 and expansion of PD-1+ TILs. (B-C) In the presence of PD-L1 on either DLBCL or nonmalignant cells, activation of TIL and expression of PD-1 are diminished, and expansion of PD-1+ TIL is inhibited. Ag, antigen; MHC, major histocompatibility complex; MΦ, macrophage; SC, stroma cell; TCR, T-cell receptor.
Regulation of PD-1 expression in T cells of the DLBCL microenvironment. (A) Direct and indirect antigen presentation by lymphoma cells and nonmalignant cells, respectively, in the absence of PD-L1 results in activation of TILs inducing expression of activation markers including PD-1 and expansion of PD-1+ TILs. (B-C) In the presence of PD-L1 on either DLBCL or nonmalignant cells, activation of TIL and expression of PD-1 are diminished, and expansion of PD-1+ TIL is inhibited. Ag, antigen; MHC, major histocompatibility complex; MΦ, macrophage; SC, stroma cell; TCR, T-cell receptor.
The pathway consisting of the PD-1 receptor (CD279) and its ligands PD-L1 and PD-L2 (B7-DC; CD273) plays a vital role in peripheral tolerance2 but also mediates inhibitory signals that compromise antitumor immunity. Targeting the PD-1/PD-L1 pathway has shown clinical efficacy in solid tumors3 but also in Hodgkin and non-Hodgkin lymphoma.4,5 In solid tumors, expression of PD-L1 in cancer cells is a prognostic factor of adverse clinical outcomes and an independent predictive factor of clinical response to therapeutic targeting of the PD-1/PD-L1 checkpoint.3,6
Kiyasu et al assessed the expression of PD-L1 in 1253 DLBCL samples using a double-immunostaining technique identifying PD-L1 and PAX-5 together in order to determine whether PD-L1 is expressed on lymphoma cells (a pattern defined as PD-L1+ DLBCL) or other cells of the tumor microenvironment (a pattern defined as microenvironmental PD-L1 [mPD-L1]+ DLBCL). The study also investigated the expression of PD-1 in TILs and assessed whether cell-specific expression of PD-L1 in the DLBCL microenvironment correlated with PD-1 expression in TILs. These features were also analyzed in conjunction with clinical outcomes.
This work resulted in several novel observations. Expression of PD-L1 in PD-L1+ DLBCL or mPD-L1+ DLBCL is associated with the nongerminal center B (GCB) type of DLBCL and Epstein-Barr virus positivity. The number of PD-1+ TILs is lower in DLBCL with PD-L1 expression on malignant or nonmalignant cells of the tumor, compared with GCB-type DLBCL, which has low prevalence of PD-L1 expression. Importantly, the low number of PD-1+ TILs correlates with the presence of B symptoms, extranodal sites, and bulky masses. In addition, patients with PD-L1+ DLBCL have inferior overall survival than patients with PD-L1− DLBCL. Moreover, lymphoma-related death, consistent with disease progression, is more frequent in patients with PD-L1+ DLBCL. In contrast, there is no significant difference in overall survival between mPD-L1+ and mPD-L1− DLBCL.
These observations reveal the significance of identifying the cell-specific expression of PD-L1 in the DLBCL microenvironment. In many cancer types, tumor-specific expression of PD-L1 is a significant determinant of adverse prognosis and a regulator of PD-1-mediated immunosuppression. In such tumors, the number of PD-1+ TILs positively correlates with tumor-specific PD-L1 expression and is a poor prognostic factor.7 In contrast to these observations in solid tumors, the presence of a high number of PD-1+ TILs is a favorable prognostic factor in patients with DLBCL and follicular lymphoma, whereas a low number of PD-1+ TILs is associated with a higher risk of histologic transformation.8,9
The study by Kiyasu et al provides new insights regarding the expression of the PD-1/PD-L1 pathway components and their implications in DLBCL. An important point that should be taken into consideration when analyzing the significance of PD-1 expression in TILs of B-cell malignancies is that PD-1 is normally expressed at high levels on germinal center follicular helper T cells.10 Therefore, PD-1+ TILs are expected to be more abundant in GCB-type DLBCL. Indeed, quantitative analysis determined that higher numbers of PD-1+ TILs are associated with GCB-type DLBCL.1 These findings suggest that in DLBCL the number of PD-1+ TILs might not reflect tumor-mediated T-cell exhaustion but might be simply indicative of the lymphoma cell origin. Consistent with this hypothesis, high numbers of PD-1+ TILs are associated with better prognosis, and this feature is not affected by the presence of PD-L1 in DLBCL or nonmalignant cells of the lymphoma microenvironment. In contrast, within the patient group with low numbers of PD-1+ TILs, expression of PD-L1 is associated with worse prognosis compared with the group that is negative for DLBCL PD-L1 or mPD-L1.1
Expression of PD-1 in TILs of DLBCL might also be regulated by other mechanisms. Tumor antigens can be presented to T cells by direct presentation by tumor cells and by indirect presentation by antigen-presenting cells (APCs). Because B cells have natural APC function, it is conceivable that lymphoma B cells might function as APCs. Thus, both lymphoma cells and APCs of the DLBCL microenvironment may induce activation of TILs, leading to the expression of activation markers, including PD-1. Such effects would be less pronounced in the presence of PD-L1, which inhibits T-cell activation and expansion (see figure). Consistent with this hypothesis, the number of PD-1+ TILs is significantly lower in DLBCL that expresses high levels of PD-L1 compared with DLBCL with low PD-L1 expression in the tumor microenvironment.1
An important finding of the study is that expression of PD-L1 on lymphoma cells but not on other cells of the DLBCL microenvironment has a significant impact on clinical outcomes. These results are reminiscent of previous observations in patients with solid tumors, where tumor-specific expression of PD-L1 is a negative prognostic determinant, and suggest that the PD-1/PD-L1 pathway may preferentially mediate its immunosuppressive effects during direct presentation of tumor antigens. However, in contrast to solid tumors, PD-L1+ DLBCLs have low numbers of PD-1+ TILs. It will be critical to determine whether anti-PD-L1 blocking antibodies might lead to superior outcomes as compared with anti-PD-1 antibodies in this subgroup of DLBCL patients. Because mPD-L1 expression does not have an impact on survival, it will be important to determine whether targeting the PD-1/PD-L1 pathway has any therapeutic benefit in this patient subgroup. Thus, identification of cell-specific expression of PD-L1 in DLBCL might have prognostic significance but might also guide treatment decisions. The present study opens new avenues in the research of PD-1/PD-L1 biology in lymphomas and suggests that the regulation of this pathway is distinct and more complex in the cellular microenvironment of B-cell malignancies than in solid tumors.
Conflict-of-interest disclosure: The author declares no competing financial interests.