In this issue of Blood, Wang and colleagues report results of the first ever conducted prospective clinical trial on salvage treatment of hemophagocytic lymphohistiocytosis (HLH) in adult patients (aHLH). The trial was conducted as a multicenter trial in 6 hematology centers in Beijing, China, where 63 treatment-refractory patients were enrolled within 12 months.1
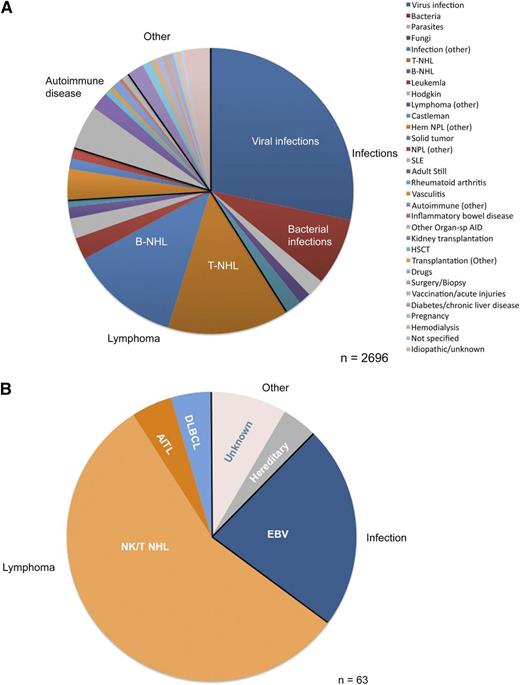
Underlying conditions and trigger diseases for the development of aHLH: comparison of (A) the global perspective, summarized by Ramos-Casals et al,6 and (B) the highly selected refractory aHLH cohort studied by Wang et al.1 Of note, given the restricted composition of trigger diseases, the prospectively designed salvage trial for aHLH with DEP regimen achieved meaningful results, whereas a one-size-fits-all treatment algorithm is not appropriate for all potential aHLH patients, as causes and trigger diseases are highly heterogeneous, requiring individualized, risk-adapted treatment.5 Hem NPL, hematologic neoplasia; HSCT, hematopoietic stem cell transplantation; NPL, neoplasia; Organ-sp AID, organ-specific autoimmune disease; SLE, systemic lupus erythematosus.
Underlying conditions and trigger diseases for the development of aHLH: comparison of (A) the global perspective, summarized by Ramos-Casals et al,6 and (B) the highly selected refractory aHLH cohort studied by Wang et al.1 Of note, given the restricted composition of trigger diseases, the prospectively designed salvage trial for aHLH with DEP regimen achieved meaningful results, whereas a one-size-fits-all treatment algorithm is not appropriate for all potential aHLH patients, as causes and trigger diseases are highly heterogeneous, requiring individualized, risk-adapted treatment.5 Hem NPL, hematologic neoplasia; HSCT, hematopoietic stem cell transplantation; NPL, neoplasia; Organ-sp AID, organ-specific autoimmune disease; SLE, systemic lupus erythematosus.
For years, HLH has been studied almost exclusively in the pediatric community, and adult hematologists have adopted the pediatric recommendations for diagnosis and treatment.2 The HLH diagnostic criteria include fever, hepatosplenomegaly, cytopenia, elevation of ferritin, soluble CD25 (soluble interleukin 2 receptor), triglycerides or depletion of fibrinogen, low/absent natural killer (NK)-cell activity, and hemophagocytosis. Five of 8 criteria must be met for the diagnosis of HLH. These criteria were developed for the prospective clinical trials of The Histiocyte Society’s protocols HLH-94 and HLH-2004,3 which included toddlers, children, and adolescents <18 years of age with HLH.3 Children are much more frequently affected by hereditary or “primary” HLH compared with adults, requiring intensive HLH-directed treatment with consolidating allogeneic stem cell transplantation. The HLH-94 protocol is a true success story as it achieved 5-year survival improvement from <5% up to >50%.4 Initial treatment consists of etoposide (VP-16) 150 mg/m2 twice weekly with dexamethasone (dex) 10 mg/m2 daily for 2 weeks with subsequent weekly etoposide infusions and tapered dex for a total of 2 months. The initial treatment phase is followed by a maintenance or continuous treatment phase that in addition to VP-16 and dex pulses contains cyclosporine A (CSA) aiming at plasma trough levels of 200 μg/L. The maintenance/continuation treatment phase was specifically designed for familial HLH and for bridging to allogeneic stem cell transplantation. Because there is still a significant failure rate of >20%, HLH-2004 added CSA to the initial treatment phase to better control cytokine storm and T-cell proliferation. However, the triple drug induction with VP-16/dex/CSA produces overt neurotoxicity. Hence, current recommendations favor VP-16/dex as induction, followed by CSA maintenance.5
In adults, management of HLH is commonly complicated by a prolonged time to diagnosis.6 As HLH is not itself a disease, but rather an excessive immune response with cytokine storm and proliferation of macrophages and CD8+ T lymphocytes in a background of immunosuppression, malignant disease, or infection, these patients are the sickest individuals seen on an adult hematology ward or intensive care unit. Identifying the underlying disease is critical for successful treatment, as disease-specific therapy can be the key to controlling aHLH.5,7 However, to achieve a state of stable multiorgan function prior to initiation of disease-specific therapy, an HLH-specific pretreatment is required in most instances. Henter et al have proposed a modified regimen for aHLH, using weekly etoposide at reduced dose (50-100 mg/m2), as pediatric etoposide doses in adults carry the risk of prolonged myelosuppression followed by life-threatening infections.8
Wang and colleagues chose to target HLH-induced inflammation using the initial drug schedule of the HLH-94 protocol, and switched to a salvage protocol for those not achieving partial remission after 2 weeks of treatment.1 Treatment failure was defined as failure to control HLH symptoms and laboratory parameters after 2 weeks of HLH-94 induction. The doxorubicin-etoposide-methylprednisolone (DEP) regimen contains pulse methylprednisolone, weekly etoposide, and liposomal doxorubicin. It achieved an overall response of 76.2%, which seems promising. This regimen is the first salvage treatment with a documented response rate in aHLH. What are the caveats to consider? The Beijing trial cohort consisted of patients with lymphoma-associated HLH (46%), Epstein-Barr virus (EBV)-triggered HLH (35%), familial HLH (6%), and cases of aHLH with unknown trigger or underlying disease (13%) (see figure). The lymphoma cohort had 86% NK/T-cell lymphomas, 2% diffuse large B-cell lymphomas (DLBCLs), and 2% angioimmunoblastic T-cell lymphomas (AITLs). It is therefore fair to say that the DEP cohort in essence is an EBV and NK/T-cell lymphoma cohort (79%), which are the classical major Asian HLH candidates.6 A salvage aHLH cohort in European or Northern American countries would very likely look much different with far fewer NK/T-cell lymphomas, more AITL and B-cell lymphomas, and more diverse triggering viruses like cytomegalovirus, varicella- zoster virus, or HIV.6 The Beijing cohort represents a selected population of refractory aHLH patients, which was highly sensitive to DEP. One can assume equal responsiveness of other lymphoma entities, but should be careful in treatment of refractory aHLH patients with other, anthracycline nonsensitive malignancies. A review on global HLH triggers and underlying diseases found a 14% T-cell non-Hodgkin lymphoma (T-NHL) fraction combined with a 12% B-cell non-Hodgkin lymphoma (B-NHL) fraction.6
In EBV-HLH, preclinical data did not suggest activity of doxorubicin. Yet, as patients were refractory to etoposide, one can only speculate that use of liposomal doxorubicin is key to enrichment and action in the macrophage compartment. Others have reported anti–CD20-directed treatment of EBV-HLH to stop EBV replication and sustained CD8+ T-cell activation by B-cell depletion.9 The study group in Beijing had chosen not to use rituximab, as endemic EBV replication in T cells protects the EBV reservoir from rituximab in Asia.
Wang and colleagues report a remarkably high 85% detection rate of aHLH causes (including hereditary aHLH [6%]), and leave only 13% of patients without detected trigger. This points to the fact that this network of specialized HLH centers has established a standardized diagnostic workup including functional cellular assays and molecular testing, explaining the high diagnostic vigilance for aHLH in Beijing.10 Time to diagnosis and treatment has been shown to impact prognosis in the pediatric setting. Rapid initiation of treatment to prevent irreversible organ damage seems of equal importance in aHLH.5 Thus, Wang et al have demonstrated a key factor for improving the still grave prognosis of aHLH patients: collaborate within an aHLH network and treat carefully selected patients within prospectively designed protocols. Looking at all potential causes of refractory HLH in adults, a “one-size-fits-all” protocol would be dangerous. A risk and disease-based treatment algorithm is proposed to protect aHLH patients from harmful, nonspecific treatment.5 The local situation in Beijing seems ideal for this advance in aHLH research, and the authors can be congratulated on this achievement.
Conflict-of-interest disclosure: The author declares no competing financial interests.