In this issue of Blood, Gilbert et al present evidence that factor VIII (fVIII) binding to thrombin-activated platelets is mediated by fibrin, in contrast to the phosphatidylserine (PS)-mediated binding to platelets activated in the presence of agonists.1
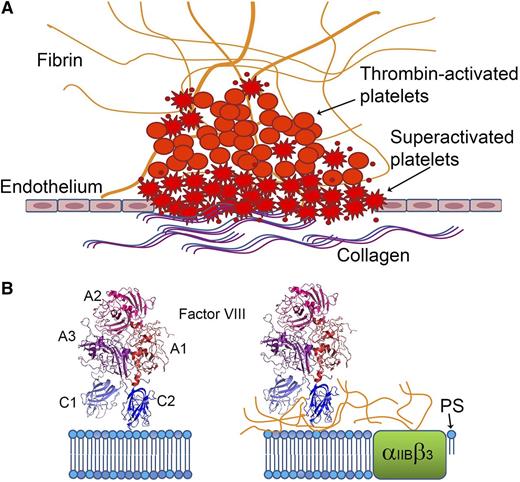
fVIII shows distinct binding characteristics when bound to platelets activated by thrombin plus agonists (“superactivated platelets”), such as collagen or A23187 vs by thrombin alone. (A) Platelets activated in the presence of collagen or other agonists undergo shape changes, expose more PS, and bind more coagulation factors. (B) PS comprises most of the fVIII-binding surfaces on superactivated platelets (left), whereas binding to less-activated platelets generated by thrombin alone is mediated by fibrin (right).
fVIII shows distinct binding characteristics when bound to platelets activated by thrombin plus agonists (“superactivated platelets”), such as collagen or A23187 vs by thrombin alone. (A) Platelets activated in the presence of collagen or other agonists undergo shape changes, expose more PS, and bind more coagulation factors. (B) PS comprises most of the fVIII-binding surfaces on superactivated platelets (left), whereas binding to less-activated platelets generated by thrombin alone is mediated by fibrin (right).
Every student of hematology and every clinical coagulation laboratory knows that a source of PS, a negatively charged phospholipid that becomes exposed on the surface of activated platelets and platelet-derived microparticles, is required for standard blood clotting assays such as the activated partial thromboplastin time (aPTT). Textbook figures illustrating the extrinsic and intrinsic coagulation pathways show “islands” of factor-cofactor substrate proteins floating on a phospholipid bilayer representation of the membrane surface. fVIII binds via its positively charged C2 domain to activated platelets, microparticles, and synthetic phospholipid vesicles that expose the PS head group, and the C1 domain has also been shown to play a role in platelet binding.2 Crystal structures and molecular models indicated that this binding involves both electrostatic and hydrophobic fVIII-membrane interactions,3 and this was confirmed by subsequent site-directed mutagenesis of membrane-binding loops in the C2 domain.4 However, there is more to the story. Early studies by Nesheim et al indicated that factor V (fV) and fVIII bind to nonoverlapping sites on thrombin-activated platelets.5 This in turn suggested a role for additional platelet membrane components in physiological binding and cofactor activities of fV and fVIII. Platelet activation enables the integrin αIIBβ3 to bind fibrin, and fVIII binding to thrombin-activated platelets increases when fibrin engages αIIBβ3.6 Thus, membrane and/or membrane-bound proteins participate in fVIII attachment and hence in the activation of factor X to Xa by factor IXa, accelerated by its cofactor fVIIIa. In other words, the “tenase” complex is more complex than initial simplified models of coagulation suggested.
Gilbert et al now present evidence that fVIII-binding sites on thrombin-activated platelets have markedly different fVIII-binding characteristics compared with sites on PS-containing phospholipid vesicles and on platelets activated in the presence of stronger agonists such as the calcium ionophore, A23187.1 PS exposure on the surface of platelets stimulated with thrombin plus A23187 or physiological agonists such as collagen is higher than PS exposure following stimulation with similar levels of thrombin alone. Indeed, “superactivated” platelets are a distinct and clinically important subset of the heterogeneous partially and fully activated platelet population. Their characteristics include cellular shape changes, clustering at collagen surfaces, prolonged calcium influx, inactivated αIIBβ3, binding of higher levels of coagulation factors, and enhanced procoagulant activity.7 Thrombin-activated platelets bind lactadherin, a PS-binding protein homologous to the C domains of fVIII and fV, whereas only superactivated platelets have sufficient PS exposure to also bind annexin. Studies of thrombus formation have localized annexin-stained platelets to the wound surface and scattered throughout the thrombus, whereas most of the platelets bound only lactadherin8 (see figure). In the present study, lactadherin inhibited fVIII binding to platelets activated by thrombin + A23187 in a dose-dependent manner but did not significantly block fVIII binding to thrombin-activated platelets. An fVIII mutant with alanine substitutions at a membrane-binding loop bound to approximately half as many sites on thrombin-activated platelets as wild-type fVIII, whereas its binding to platelets activated by thrombin + A23187 was reduced dramatically. fVIII binding was demonstrated to bead-immobilized soluble fibrin; this binding was inhibited by von Willebrand factor (VWF), the chaperone protein that circulates bound to fVIII. Monoclonal antibodies recognizing two nonoverlapping regions on the C2 domain surface blocked fVIII binding to fibrin-coated beads and reduced fVIII binding to thrombin-stimulated platelets as well as tenase activity supported by these platelets. The addition of soluble fibrin doubled the tenase activity supported by phospholipid vesicles, whereas this acceleration was lost when either antibody was added before the reaction was initiated. These anti-C2 antibodies also blocked fVIII activity in assays carried out with reconstituted platelet-rich plasma, whereas chromogenic and aPTT assays in the presence and absence of VWF showed variable inhibition of fVIII activity. Thus, sites on fVIII that bind PS-containing surfaces, VWF, and fibrin appear to partially overlap and they include the C2 domain (see figure).
Several caveats can be mentioned, all of which point toward future research questions. This study has localized a region of fVIII that binds to fibrin (the C2 domain), whereas the fibrin site that binds fVIII remains undefined. This site appears to be rare (fVIII activity was maximal at a fibrin monomer:fVIII ratio of 30:1), it is not the γ′ domain, and fVIII binding was not prevented by anti-fibrin(ogen) antibodies. The binding site for fVIII may be conformational or even an as yet undefined component that associates with fibrin. As the authors are careful to state, PS may still play an important role in fVIII attachment to thrombin-activated platelets (eg, by affecting its conformation and activity). The relative importance of PS and fibrin in fVIII activity supported by platelet-derived microparticles remains to be elucidated, as does the possible interaction of fVIII(a) with fibrin generated at nonplatelet surfaces (eg, activated endothelium and subendothelial tissue following different types of vascular injury).
An intriguing corollary to these experimental results is the hypothesis that novel fVIII activity assays carried out with platelet-rich plasma may reflect endogenous fVIII activity in patients more accurately, and over a larger dynamic range, than standard chromogenic and clotting assays. Such assays (which would be significantly more complex due to inclusion of platelets and hence issues of defining/standardizing platelet activation potential and status) could be useful in some specialized but important clinical settings (eg, measuring fVIII activity in moderate or severe hemophilia A plasma or in the presence of neutralizing antibodies). The dynamic range and sensitivity to antibody inhibition shown in the preliminary assays reported here provide encouragement for further exploration and refinement of this type of assay.
The opinions or assertions contained herein are those of the author and are not to be construed as official or reflecting the views of the Department of Defense or the Uniformed Services University of the Health Sciences.
Conflict-of-interest disclosure: The author declares no competing financial interests.