In utero transplantation has been successful in selected congenital immunodeficiencies. However, therapeutic levels of engraftment needed for hemoglobin (Hb) disorders have not been achieved.1 In this issue of Blood, Peranteau et al demonstrate a strategy to achieving tolerance induction for later therapeutic transplantation.2 After establishing immunologic tolerance in mouse models of Hb disorders, the investigators injected a “booster” dose of histocompatibility complex-mismatched donor marrow in the tolerant sickle or thalassemia (Thal) post utero recipients after a nonmyeloablative dose of total body irradiation. The booster dose was sufficient to eliminate the phenotypic expression of the underlying Hb disorder by virtue of an enrichment of donor red blood cells (RBCs) in the recipients’ blood, resulting in mixed chimerism, which if stable, has been curative after conventional allografting (see figure).3
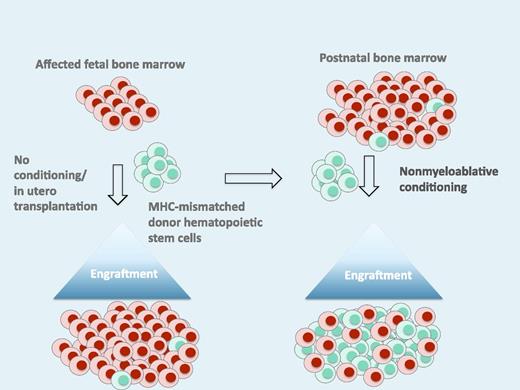
This schematic illustrates the induction of tolerance after in utero transplantation, which is followed by a second “booster” transplant after birth with the same donor to establish donor-host mixed chimerism. The level of chimerism after the “booster” is sufficient for a clinical effect. MHC, major histocompatibility complex.
This schematic illustrates the induction of tolerance after in utero transplantation, which is followed by a second “booster” transplant after birth with the same donor to establish donor-host mixed chimerism. The level of chimerism after the “booster” is sufficient for a clinical effect. MHC, major histocompatibility complex.
In the quest to develop in utero transplantation for Hb disorders, the results of these preclinical experiments are significant for several reasons. Broadly speaking, the principal barriers to hematopoietic cell transplantation (HCT) in Thal and sickle cell disease (SCD) are donor availability, and concerns about toxicities associated with conventional allogeneic transplantation, which include death, long-term disability from chronic graft-versus-host disease (GVHD), and risk of infertility.4,5 The strategy outlined by Peranteau et al addresses these issues. First, the use of a human leukocyte antigen (HLA) haploidentical donor for in utero transplantation essentially eliminates the problem of donor availability. In addition, it is feasible and perhaps even preferable to use an HLA-haploidentical maternal donor to establish donor tolerance in the fetus. The lowered immunologic barrier to donor engraftment should enable a reduced intensity, chemotherapy-based preparative regimen that avoids the risks of radiation exposure to the developing newborn brain, yet still can establish donor mixed chimerism that is sufficient for a clinical benefit. The ideal conditioning regimen would not cause transplant-related toxicities, such as infections associated with a long period of cytopenias after HCT and reduce or eliminate the risk of infertility after transplantation.
The principal challenges to translating these preclinical findings to the clinic are related to risks of in utero manipulations and of in utero GVHD. Because the vast majority of children affected by hemoglobinopathies survive to adulthood as a result of very good supportive care, safety of the in utero procedure will be critical. The technical aspects of in utero transplantation involve injection into the umbilical cord vasculature, which is routinely performed for in utero transfusions. However, transplantation in the early second trimester may be more challenging given the size of the fetus and carry additional procedural risks. With respect to the potential for prenatal GVHD, one interesting finding observed in this and in an earlier canine study from this group is the absence of GVHD in spite of administering a large T-cell dose (2.5 to 4 × 108/kg).6 It is possible that immunologic immaturity or plasticity confers resistance to GVHD and establishes tolerance, although specific mechanisms are not known. These reports suggest that transplantation into the fetal host may reduce GVHD and induce tolerance, which together allow for a less intensive preparative regimen after birth.
The developing immune system in the fetal period creates a unique immunologic opportunity to induce tolerance to transplanted cells. Although the human fetus has mature α/β T cells by the early second trimester, the fetal T-cell lineage may be more biased toward immune tolerance7 and may be particularly prone to tolerating maternal cells, given the presence of microchimeric maternal cells at baseline in the fetal circulation.8 Thus, transplantation of maternal hematopoietic stem cells into the very early gestation fetus to induce donor-specific tolerance, followed by postnatal booster transplantation may be a viable strategy for the prenatal treatment of hemoglobinopathies. Other improvements to the strategy, such as creating space in the host bone marrow niche9 or improving the competitive advantage of the transplanted cells10 may be needed to achieve levels high enough to tolerize after the initial in utero donor cell inoculum. This report is therefore a critical advance supporting the promise of this emerging branch of fetal therapy.
Where to begin clinical investigation of in utero transplantation for Hb disorders will require careful thought. The variable and unpredictable phenotype of SCD in children might not lend itself easily to testing an intensive therapy in a presymptomatic fetus. Conversely, Thal major has a more predictable genotype/phenotype relationship, and there is likely to be interest in pursuing a curative therapy that might bypass the need for regular RBC transfusions in childhood. The optimal target, however, might be α-Thal major, which is typically accompanied by fetal demise in the absence of in utero RBC transfusions. At a minimum, progress in this area is very likely to expand the breadth of curative choices for hemoglobinopathies.
Conflict-of-interest disclosure: M.C.W. is Medical Director of ViaCord Processing Laboratory and AllCells, Inc. T.C.M. declares no competing financial interests.