In this issue of Blood, Tawana et al describe 24 patients with acute myeloid leukemia (AML) within 10 families with germline, ie, hereditary, mutations in the CCAAT/enhancer binding protein α (CEBPA) gene. Distinct biology and clinical outcomes, including unique patterns of “relapse,” are identified.1
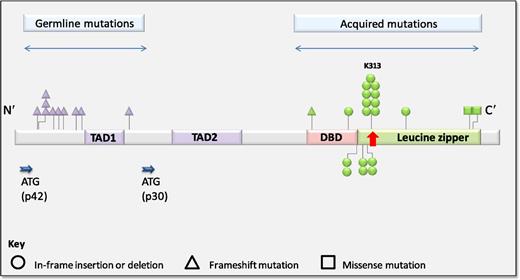
Distribution of germline and acquired CEBPA mutations in familial AML. See Figure 2A in the article by Tawana et al that begins on page 1214.
Distribution of germline and acquired CEBPA mutations in familial AML. See Figure 2A in the article by Tawana et al that begins on page 1214.
C/EBPα is a key hematopoietic transcription factor involved in lineage-specific myeloid differentiation. Somatic mutations in the gene encoding C/EBPα (CEBPA) occur in ∼10% of AMLs and contribute to leukemic transformation through impaired myeloid differentiation.2
Pathogenic CEBPA mutations occur primarily within 2 discrete regions (see figure). N-terminal mutations are characteristically frameshift insertions or deletions, leading to forced translation of an alternate 30-kDa protein from within an internal start site, which exerts dominant-negative effects on the full-length CEBPA protein. C-terminal in-frame insertions/deletions occur within the DNA-binding or leucine zipper domains and disrupt DNA binding and dimerization.3
In 2004, the first family with a germline CEBPA mutation was described, when 3 family members diagnosed with AML demonstrated an identical N-terminal CEBPA deletion in all diagnostic tumors and normal/remission samples.4 This autosomal dominant syndrome is now formally designated “familial AML with mutated CEBPA”.5 Although several CEBPA-mutated families have been previously reported, genetic events underlying subsequent AML development and the clinical course of these patients remain ill defined.
This collaborative multicenter study details the clinicopathologic features of 24 AML patients from 10 families with germline CEBPA mutations and sheds more light on this unique inherited syndrome. First, Tawana et al confirm that germline CEBPA mutations occur primarily within the N-terminal domain, with secondary C-terminal CEBPA mutations acquired at AML diagnosis. In 18 diagnostic tumors tested, C-terminal CEBPA mutations leading to double-mutant CEBPA (dmCEBPA) AML were universally identified. In addition, 5 of 9 exome-sequenced tumors acquired a GATA2 mutation, reinforcing a previously described and still poorly understood connection between dmCEBPA and mutated GATA2.6
Furthermore, Tawana et al establish the highly penetrant nature of germline CEBPA mutations leading to subsequent AML development. AML developed at a median age of 24 years (range, 1.8-46 years) in affected individuals. Compared with other inherited leukemia syndromes such as RUNX1-mutated familial platelet disorder/propensity to acute leukemia with ∼35% lifetime risk of developing hematologic malignancy,7 the sparse available literature on hereditary CEBPA suggests 100% AML penetrance. In this study, 3 young asymptomatic carriers (ages 19, 24, and 41 years) are described; all other affected individuals have developed AML.
An important discovery comes from molecular sequencing of diagnostic and relapsed AML samples from 5 patients. In these well-annotated tumors, the relapse was clonally distinct from the diagnostic AML, including different acquired CEBPA mutations in all cases, indicating the recurrences are second primary leukemias rather than relapsed disease. This highlights again the preferential specificity of acquired CEBPA mutations leading to dmCEBPA at the time of AML and helps explain the excellent outcomes observed even in the relapsed setting. With a >90% remission rate and a >50% cumulative incidence of relapse, sustained remissions after the third and even fourth relapses were observed. Of 7 deaths occurring after AML recurrence, 5 occurred in remission due to treatment or transplant-related toxicity. In this maturing era of individualized cancer therapy, attention to this striking chemosensitivity (with 1 patient obtaining a 45-year remission with steroids and mercaptopurine alone) is noteworthy and suggests treatment-related toxicity as the primary cause of mortality in familial CEBPA-mutated AML patients.
Although much has been elucidated through this detailed examination of familial CEBPA-mutated AML, new challenges and questions are brought to the forefront. In particular, given the increasing use of gene panels for leukemia diagnosis and disease monitoring, coupled with the difficulty of obtaining germline samples in patients with hematologic malignancies, there is an emerging need to address possible germline findings identified by these sequencing efforts.
What is the overall prevalence of hereditary CEBPA-mutated families? Are they as rare as predicted from less than a dozen families described in a decade? In fact, current evidence suggests familial AML may be significantly more frequent. A recent study of 187 AML patients identified 18 (9.6%) CEBPA-mutated patients, with 2 of 18 (11%) having germline CEBPA mutations.8 (On review, additional members of both families had been affected by AML.) Another study of 1182 adults with diploid AML identified 151 (12.8%) patients with CEBPA mutations. Available germline testing in 71 patients demonstrated familial CEBPA mutations in 5 (7%; 4 dmCEBPA and 1 single-mutant CEBPA). These data suggest additional families may be ascertained by close attention to family history and careful evaluation of presumed somatic CEBPA mutations.
With a predicted frequency of ∼10% in presumed somatic CEBPA-mutated patients, should all patients with CEBPA mutations be referred for genetic counseling? Or perhaps only those with dmCEBPA, a persistently detected CEBPA mutation in remission, or a family history of leukemia in first-degree relatives? Further studies are needed to illuminate the answers to these questions. Nonetheless, an effort to screen affected family members also serves the critical function of determining appropriate donors for allogeneic transplantation.
As there are no prodromal clinical signs, symptoms, or antecedent cytopenias in affected individuals prior to AML development, what should be the appropriate method of screening and surveillance? Expert opinion recommends a baseline bone marrow aspirate and biopsy, with regular clinic visits and complete blood counts.9 Undoubtedly, the focus should be on early detection and prevention strategies, and with the frequency of acquired CEBPA and GATA2 mutations at the time of AML, current strategies should focus on methods to detect secondary clonal events, with future efforts focusing on methods to promote normal myeloid differentiation.
Although hematologic malignancies have long been considered sporadic in nature, there is a growing awareness of the genetic basis of various inherited leukemia predispositions, including familial AML with mutated CEBPA. Yet evaluation for these hereditary syndromes is routinely underperformed, and improved recognition is needed. The collaborative work of Tawana et al increases the clinical awareness and understanding of this unique leukemia predisposition syndrome. Future studies should help define the frequency and clinical indicators to predict those patients most likely to have germline predispositions: to prompt genetic testing and counseling, optimize medical care, and pave the way for novel interventions to reduce their lifetime risk of leukemia.
Conflict-of-interest disclosure: The author declares no competing financial interests.