Key Points
DFX-DFO combination followed by DFX monotherapy led to a meaningful decrease in myocardial and liver iron in severe siderosis patients.
Substantial liver iron reduction may be helpful in patients needing rapid control of liver iron (eg, pretransplant or planned pregnancy).
Abstract
Deferasirox (DFX) monotherapy is effective for reducing myocardial and liver iron concentrations (LIC), although some patients may require intensive chelation for a limited duration. HYPERION, an open-label single-arm prospective phase 2 study, evaluated combination DFX-deferoxamine (DFO) in patients with severe transfusional myocardial siderosis (myocardial [m] T2* 5-<10 ms; left ventricular ejection fraction [LVEF] ≥56%) followed by optional switch to DFX monotherapy when achieving mT2* >10 ms. Mean dose was 30.5 mg/kg per day DFX and 36.3 mg/kg per day DFO on a 5-day regimen. Geometric mean mT2* ratios (Gmeanmonth12/24/Gmeanbaseline) were 1.09 and 1.30, respectively, increasing from 7.2 ms at baseline (n = 60) to 7.7 ms at 12 (n = 52) and 9.5 ms at 24 months (n = 36). Patients (17 of 60; 28.3%) achieved mT2* ≥10 ms and ≥10% increase from baseline at month 24; 15 switched to monotherapy during the study based on favorable mT2*. LIC decreased substantially from a baseline of 33.4 to 12.8 mg Fe/g dry weight at month 24 (−52%). LVEF remained stable with no new arrhythmias/cardiac failure. Five patients discontinued with mT2* <5 ms and 1 died (suspected central nervous system infection). Safety was consistent with established monotherapies. Results show clinically meaningful improvements in mT2* in about one-third of patients remaining on treatment at month 24, alongside rapid decreases in LIC in this heavily iron-overloaded, difficult-to-treat population. Combination therapy may be useful when rapid LIC reduction is required, regardless of myocardial iron overload. This trial was registered at www.clinicaltrials.gov as #NCT01254227.
Introduction
Patients with transfusional iron overload are at risk of developing myocardial iron deposition. Severe myocardial iron overload (myocardial [m] T2* <10 ms) is associated with a substantially increased risk of heart failure.1-3 As such, some patients may require intensified chelation4 for a limited duration to expedite transition from a high- to low-risk status for heart failure (mT2* <10 ms to ≥10 ms).1
Significant reduction of total body iron overload, including clinically relevant decreases in myocardial iron, has been demonstrated with deferasirox (DFX) or deferoxamine (DFO) monotherapy in transfusion-dependent thalassemia patients with mild, moderate, and severe myocardial iron overload.5-11 However, suboptimal myocardial responses to DFX have been identified in patients with severe myocardial and liver iron deposition, who may, therefore, require more aggressive therapy.12 Long-term continuous IV DFO monotherapy has been explored in patients at high risk of cardiac toxicity, although compliance remains an issue.13 Combination therapy with deferiprone and DFO has also been reported in both prospective and retrospective studies and shown substantial improvement in myocardial iron burden.14-17 Small studies using combination DFX-DFO therapy have shown a rapid reduction in systemic and myocardial iron with no increase in toxicity.18-21 However, few patients assessed with any combination therapy to date have had severe total body iron burden as reflected by significant elevations in both liver and myocardial iron.
Combined modalities may increase the rate of iron removal from both the liver and myocardial tissues and further investigation into combination regimens could permit the use of lower doses of individual chelators vs monotherapy, thereby reducing the potential for unwanted side effects. Improved definition of the clinical circumstances that may benefit most from combination therapy will help optimize a tailored approach to patient treatment. As thalassemia patients require lifelong chelation therapy, patient satisfaction and adherence should also be considered.22 Ultimately, the objective should be to maintain a normalized iron burden with oral monotherapy instead of cumbersome combination therapy. However, greater understanding of when patients can switch from combination to monotherapy is needed. The current study was designed to evaluate a treatment regimen of combined DFX and DFO therapy in patients with severe transfusional myocardial siderosis, followed by DFX monotherapy when patients reached a lower risk status for heart failure, over a 24-month period. The primary objective was to evaluate the effect of this treatment modality on myocardial iron content as depicted by change in mT2* at month 12.
Methods
Patients
HYPERION was conducted between January 2011 and November 2013. Inclusion criteria were:
Patients aged ≥10 years with β-thalassemia major, Diamond-Blackfan anemia (DBA), or congenital sideroblastic anemia on chronic transfusion therapy with cardiovascular magnetic resonance (CMR)–measured mT2* 5 to <10 ms;
CMR-measured left ventricular ejection fraction (LVEF) ≥56%, R2-magnetic resonance imaging (MRI)–measured liver iron concentration (LIC) ≥7 mg Fe/g dry weight (dw), and serum ferritin ≥1000 ng/mL;
A lifetime history of ≥50 U of red blood cell (RBC) transfusions and transfusional requirement of ≥8 U per year of RBC.
Key exclusion criteria included:
Clinical symptoms of cardiac dysfunction;
Serum creatinine above the upper limit of normal (ULN);
Significant proteinuria (urinary protein/creatinine ratio [UPCR] ≥1.0 mg/mg) and alanine aminotransferase (ALT) levels >5× ULN, but only if LIC was <10 mg Fe/g dw.
Study design
HYPERION was a 24-month prospective, phase 2, open-label, single-arm, multicenter study. Eligible patients had a 5-day washout period followed by a starting daily dose of oral DFX 20 mg/kg in combination with infused DFO 40 mg/kg for 5 days per week for ≥8 hours per day.
Dose escalation of DFO was not permitted. DFX dose was increased to 30 and then 40 mg/kg per day at the end of months 1 and 6, respectively, unless any criteria for dose reduction or drug interruption were met (eg, drug-related adverse events [AEs]). Downward dose adjustments for both drugs were advised based on LIC, serum ferritin, renal and hepatic laboratory parameters, gastrointestinal AEs, rash, auditory and ocular abnormalities, or infection.
Patients achieving mT2* ≥10 ms with a relative mT2* increase of ≥10% from baseline after 6 months had the option to switch to DFX monotherapy (30-40 mg/kg per day). Combination therapy was resumed with the highest tolerable DFX and DFO dose if mT2* fell to <10 ms with a relative decrease of ≥10% from previous mT2* value. Both DFX and DFO were to be interrupted, with continued monitoring of LIC and mT2*, if serum ferritin was confirmed <200 ng/mL or LIC was <1.5 mg Fe/g dw.
The study was conducted in accordance with Good Clinical Practice guidelines and the Declaration of Helsinki and was approved by institutional ethics committees at participating sites. Written informed consent was obtained prior to any screening procedures.
End points
The primary efficacy end point was change in mT2* assessed by the ratio of the geometric mean (Gmean) mT2* at month 12 divided by that at baseline (Gmean12/Gmeanbaseline). A key secondary end point was the proportion of patients achieving mT2* ≥10 ms and a ≥10% relative increase from baseline at months 6, 12, 18, and 24. Other secondary end points were change in mT2* and change in LVEF at month 24, time to achieve mT2* ≥10 ms (with ≥10% relative increase from baseline), and safety assessments.
Assessments
Efficacy was analyzed according to the study protocol for all evaluable patients in the full analysis set (FAS), defined as patients who received at least 1 dose of study drug and had a baseline and postbaseline assessment within each visit window. Last observation carried forward (LOCF) was used at month 12 when analyzing mT2* data on the evaluable subset of the FAS.
mT2* and LVEF were measured by CMR23 ; LIC was measured using a validated R2 MRI technique24 at baseline and 6-monthly visits up to month 24. Serum ferritin was assessed monthly. Patients without baseline data or any postbaseline value in a visit window were excluded from analysis at that time point. Myocardial iron concentration (MIC) was derived from mT2* values based on the formula described by Carpenter et al.25
The safety set consisted of all patients who received at least 1 dose of study drug and had at least 1 postbaseline assessment. Safety was evaluated through continuous monitoring and recording of AEs, serious AEs, laboratory testing, and clinical evaluations, with biweekly assessments for the first 2 months of treatment. Both efficacy and safety assessments included patients who remained on combination therapy and those who switched to monotherapy during the study.
Statistical methods
A sample size of 45 enrolled patients was required to obtain a 2-sided 95% confidence interval (CI) for the ratio of mT2* at month 12 divided by mT2* at baseline (Gmean12/Gmeanbaseline) with a half-width of 0.065 on the log scale assuming lognormal distribution of mT2*, standard deviation (SD) of 0.2 on the log scale, and 20% dropout rate. For the primary efficacy end point, the Gmean (Gmean12/Gmeanbaseline) was presented with a 95% CI. Efficacy analyses were summarized descriptively. A post hoc analysis of the intention-to-treat (ITT) population was also performed for the end point relating to the proportion of patients achieving mT2* ≥10 ms and a ≥10% relative increase from baseline.
Results
Patients
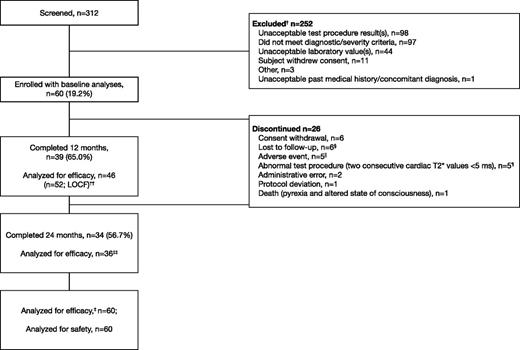
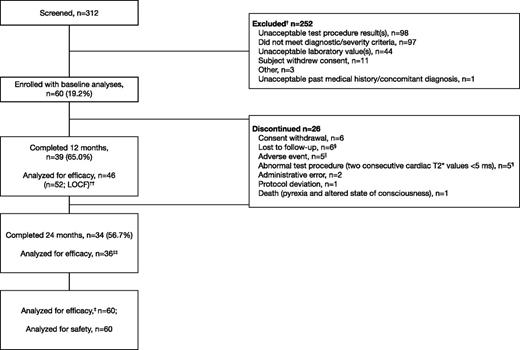
Of 312 patients screened, 60 were enrolled (59 with β-thalassemia major and 1 with DBA; Table 1). Thirty-four patients (56.7%) completed 24 months of treatment, with the majority of discontinuations in the first 12 months of the study (n = 21; Figure 1). Five patients discontinued due to confirmed mT2* <5 ms as per protocol; of these, 4 patients had mT2* <6 ms at baseline, and 1 patient had mT2* of 6.1 ms at study entry. Patients with a single assessment of mT2* <5 ms or LVEF <56% are discussed in the supplemental Data available on the Blood Web site. Other main reasons for discontinuation included loss to follow-up (n = 6) and consent withdrawal (n = 6; including personal reasons such as relocation and inability to visit the investigational site).
Patient disposition. † indicates that a patient could have multiple reasons for screening failure. ‡, The primary efficacy end point was assessed using the full analysis set. §, Five patients from Egypt and 1 patient from Italy. ‖AEs that led to discontinuation were abdominal pain (2), arthritis (1), drug rash with eosinophilia and systemic symptoms (1), and pruritus (1). ¶, Four patients had mT2* <6 ms at baseline, and 1 patient had mT2* of 6.1 ms at study entry. ††, Last available value within month 12 window included in efficacy analysis, with exception of month 12 mT2* where LOCF was used. ‡‡, Two patients were measured within the month 24 window and were included in efficacy analyses, but failed to complete the full 24 months.
Patient disposition. † indicates that a patient could have multiple reasons for screening failure. ‡, The primary efficacy end point was assessed using the full analysis set. §, Five patients from Egypt and 1 patient from Italy. ‖AEs that led to discontinuation were abdominal pain (2), arthritis (1), drug rash with eosinophilia and systemic symptoms (1), and pruritus (1). ¶, Four patients had mT2* <6 ms at baseline, and 1 patient had mT2* of 6.1 ms at study entry. ††, Last available value within month 12 window included in efficacy analysis, with exception of month 12 mT2* where LOCF was used. ‡‡, Two patients were measured within the month 24 window and were included in efficacy analyses, but failed to complete the full 24 months.
Patients had substantial iron overload at baseline; 68.3% of patients had LIC >30 mg Fe/g dw with a mean LIC of 33.4 ± 14.5 mg Fe/g dw, and Gmeanbaseline mT2* was 7.2 ms (95% CI: 6.83, 7.58). Median serum ferritin was 5551 ng/mL. All patients had a LVEF ≥ 56% at baseline; by Westwood criteria,26 most patients (85.0%) had an LVEF at or above the lower limit of normal (LLN).
Exposure to treatment and compliance.
Overall, 15 patients (25.0%) switched to DFX monotherapy at some point during the study based on favorable mT2* values; 7 patients switched in month 6, 4 in month 12, 1 in month 18, and 3 in month 24 visit windows. Two patients who first switched to monotherapy during month 6, then switched back to combination therapy during months 12 and 19, respectively. As planned in the study design, these patients are evaluated together with the patients continuing combination therapy.
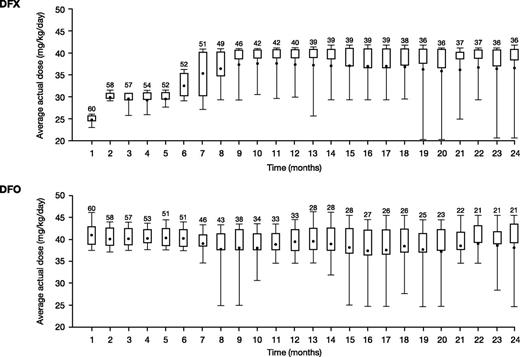
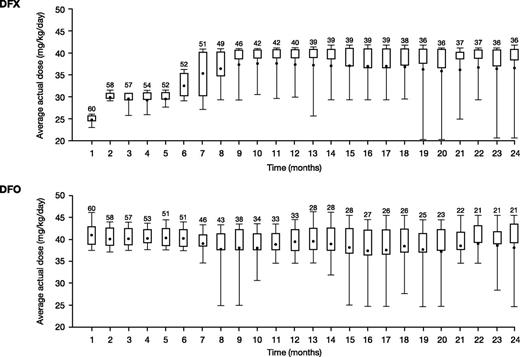
Average actual DFX and DFO doses over the first 12 months were 29.6 ± 6.3 mg/kg per day and 37.4 ± 5.8 mg/kg per day (normalized to a 5-day regimen; 26.7 ± 4.1 mg/kg per day for a 7-day regimen), respectively. Average dose was similar throughout the 24-month study (30.5 ± 7.1 mg/kg per day DFX and 36.3 ± 6.7 mg/kg per day for DFO). Monthly mean actual dose is shown in Figure 2. The median (range) duration of exposure was 719.0 days (21.0-737.0) for DFX and 407.5 days (29.0-737.0) for DFO, with a total exposure of 86.9 and 72.7 patient-years, respectively.
Mean daily dose in patients treated with DFX-DFO in combination followed by DFX monotherapy over 24 months. The length of the box represents the interquartile range (distance between the 25th and 75th percentiles); the whiskers extend to the 10th and 90th percentile. The means are presented as dots.
Mean daily dose in patients treated with DFX-DFO in combination followed by DFX monotherapy over 24 months. The length of the box represents the interquartile range (distance between the 25th and 75th percentiles); the whiskers extend to the 10th and 90th percentile. The means are presented as dots.
Of all patients enrolled (n = 60), 99.4% ± 4.9% of the planned DFX dose and 97.6% ± 12.7% of the planned DFO dose were taken. DFX or DFO treatment was interrupted at least once in 36 patients (60.0%) and 28 patients (46.7%), respectively. Interruption was most commonly due to AEs (n = 27 [45.0%] for DFX and n = 20 [33.3%] for DFO). Per-protocol interruptions due to efficacy included those for DFX, which were implemented in 2 patients (serum ferritin <200 ng/mL, LIC <3 mg Fe/g dw; n = 1 each) and for DFO in 7 patients (LIC <3 mg Fe/g dw, serum ferritin <1000 ng/mL; n = 1 each and other; n = 5). One patient interrupted both DFX and DFO as a result of LIC <1.5 mg Fe/g dw at month 18. Median (range) duration of interruption for DFX and DFO was 6.7 (1.0-57.0) and 6.6 (1.5-370.0) days, respectively.
DFX or DFO dose was reduced at least once in 32 patients (53.3%) and 37 patients (61.7%), respectively; the main reasons for dose reduction were in response to an AE (n = 26 [43.3%] and n = 19 [31.7%], respectively); per protocol (n = 4 [6.7%], n = 18 [30.0%], respectively); dosing error (n = 1 [1.7%], each) and other (n = 5 [8.3%], each). Patients could have had more than 1 reason for dose reduction.
Average iron intake
Average iron intake was <0.3 mg/kg per day for 46.7% of patients (n = 28), 0.3 to 0.5 mg/kg per day in 24 patients (40.0%), and >0.5 mg/kg per day in 11.7% of patients (n = 7) (data missing, n = 1).
Efficacy
Myocardial iron removal.
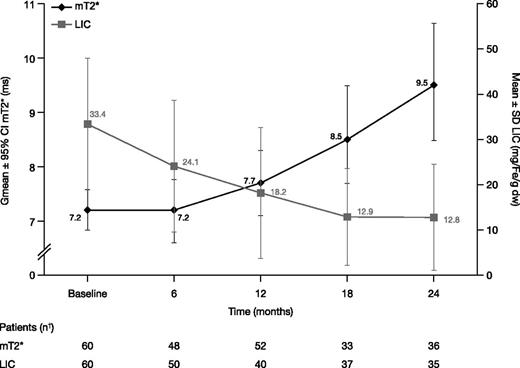
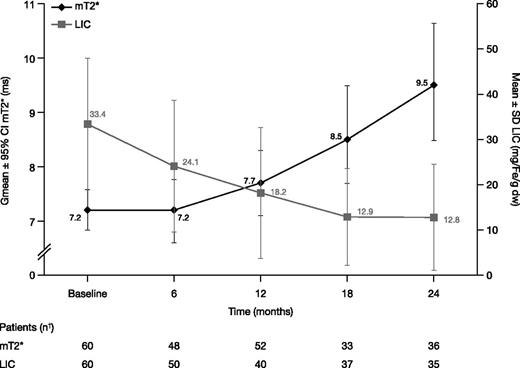
In the FAS, Gmean mT2* increased from 7.2 ms at baseline (n = 60) to 7.7 ms at month 12 (n = 52; Gmean ratio 1.09 [95% CI: 1.04, 1.15]; median change from baseline 0.7, range −3.1 to 4.5) indicating a 9% improvement from baseline. Eight patients did not have a postbaseline mT2* measurement and were therefore not evaluable at month 12. Gmean mT2* continued to increase to 9.5 [8.5, 10.6] ms at month 24 (n = 36). Ratio of Gmeans was 1.30 (95% CI: 1.17, 1.44; Figure 3), indicating a 30% improvement from baseline (median change from baseline 2.4 ms, range −2.4 to 10.0).
mT2* and LIC in patients treated with DFX-DFO in combination followed by DFX monotherapy over 24 months. † indicates that month value was the last available value within each visit window, for example, days 1 to 210, 211 to 390, 391 to 570, and 571 to 750 for months 6, 12, 18, and 24, respectively, with the exception of month 12 mT2* where last observation carried forward was used. Hence, although 34 patients completed 24 months, 36 or 35 patients had an mT2* or LIC measurement within the visit window.
mT2* and LIC in patients treated with DFX-DFO in combination followed by DFX monotherapy over 24 months. † indicates that month value was the last available value within each visit window, for example, days 1 to 210, 211 to 390, 391 to 570, and 571 to 750 for months 6, 12, 18, and 24, respectively, with the exception of month 12 mT2* where last observation carried forward was used. Hence, although 34 patients completed 24 months, 36 or 35 patients had an mT2* or LIC measurement within the visit window.
MIC remained steady from a baseline of 4.2 ± 1.0 (n = 60) to 4.3 ± 1.4 mg Fe/g dw at month 6 (n = 48), but decreased to 3.9 ± 1.4 mg Fe/g dw at month 12 (n = 46; change from baseline −0.3 ± 0.9 [95% CI: −0.62, −0.07]; −8.3%), and to 3.1 ± 1.4 mg Fe/g dw at month 24 (n = 36; −0.9 ± 1.3 [95% CI: −1.35, −0.51]), a 22.2% decrease.
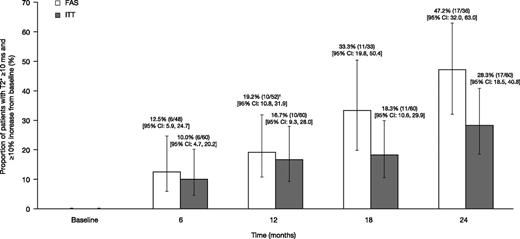
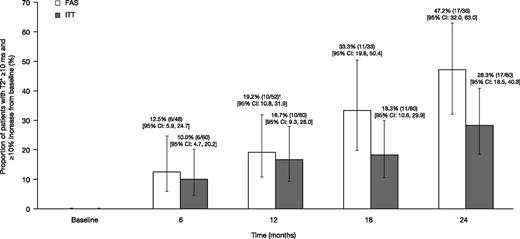
The proportion of patients achieving mT2* ≥10 ms and ≥10% relative increase from baseline, based on the FAS (evaluable patients who remained on treatment with an assessment within each visit window or LOCF at month 12) was 12.5% at month 6 (n = 6 of 48 [95% CI: 5.9, 24.7]); 19.2% (n = 10 of 52 [95% CI: 10.8, 31.9]) at month 12; 33.3% (n = 11 of 33 [95% CI: 19.8, 50.4]) at month 18; and 47.2% (n = 17 of 36 [95% CI: 32.0, 63.0]) at month 24 (Figure 4). When including all patients enrolled in a post hoc ITT analysis, the proportions were 10.0% at month 6 (n = 6 of 60 [95% CI: 4.7, 20.2]), 16.7% (n = 10 of 60 [95% CI: 9.3, 28.0]) at month 12, 18.3% (n = 11 of 60 [95% CI: 10.6, 29.9]) at month 18, and 28.3% (n = 17 of 60 [95% CI: 18.5, 40.8]) at month 24 (Figure 4). Overall, 21 patients achieved an mT2* value of ≥10 ms and a ≥10% relative increase from baseline at any time in the study, with a median time to response of 722 days.
The proportion of patients achieving mT2* ≥10 ms and ≥10% relative increase from baseline when treated with DFX-DFO combination followed by DFX monotherapy over 24 months. † indicates the last observation carried forward. FAS, full analysis set (patients who remained on treatment with an available assessment within each visit window); ITT, intent-to-treat (including all patients enrolled).
The proportion of patients achieving mT2* ≥10 ms and ≥10% relative increase from baseline when treated with DFX-DFO combination followed by DFX monotherapy over 24 months. † indicates the last observation carried forward. FAS, full analysis set (patients who remained on treatment with an available assessment within each visit window); ITT, intent-to-treat (including all patients enrolled).
Of 32 patients with baseline mT2* 6 to <10 ms and with an assessment within the month 24 visit window, 17 (53.1%) improved to mT2* ≥10 ms, 13 (40.6%) remained within the same category, and 2 patients (6.3%) worsened with mT2* <5 ms. Of 4 patients with mT2* 5 to <6 ms at baseline and with an assessment within the month 24 visit window, 3 patients improved to between 6 and <10 ms at month 24 and 1 patient worsened with mT2* <5 ms. Of 10 patients with baseline mT2* 5 to <6 ms, 3 completed the study, 4 discontinued as a result of mT2* <5 ms as per protocol, 1 was lost to follow-up, and 2 withdrew consent as a result of personal reasons.
Improvements in mT2* were observed across all baseline iron-loading subgroups examined (Table 2). Careful interpretation of findings in the lower serum ferritin subgroup in particular is needed as the sample size is small.
Liver iron removal.
Mean ± SD LIC decreased by 9.2 ± 8.7 mg Fe/g dw at month 6 (n = 50) from a baseline of 33.4 ± 14.5 mg Fe/g dw. LIC reduction continued with a 46.4% decrease (change from baseline −14.4 ± 12.1 mg Fe/g dw) at month 12 (n = 40) and a decrease of 52.3% to 12.8 ± 11.7 mg Fe/g dw at month 24 (n = 35; −17.3 ± 15.0 mg Fe/g dw; Figure 3).
Serum ferritin reduction.
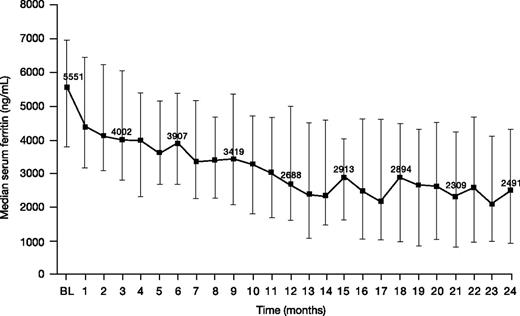
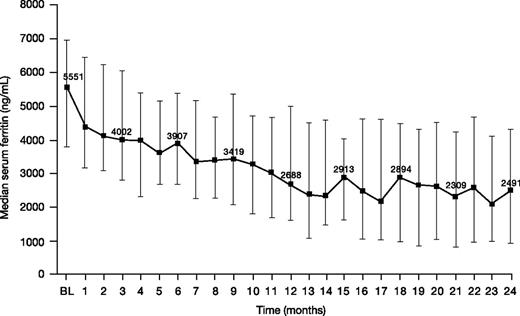
Median (range) serum ferritin decreased from 5551 (1163-11 317) ng/mL at baseline to 2491 (108-11 508) ng/mL at month 24 (n = 34; change from baseline −2064 [−5750 to 3190] ng/mL; −43.9%; Figure 5).
Serum ferritin levels in patients treated with DFX-DFO in combination followed by DFX monotherapy over 24 months. Error bars represent 25th and 75th percentiles. BL, baseline.
Serum ferritin levels in patients treated with DFX-DFO in combination followed by DFX monotherapy over 24 months. Error bars represent 25th and 75th percentiles. BL, baseline.
Cardiac function
Mean LVEF remained stable and within the normal range after 2 years of treatment (change +0.9 ± 6.0%; supplemental Figure 1). No patient discontinued as a result of LVEF <50%, as per protocol, and none developed clinical heart disease or experienced arrhythmia during the study.
Safety parameters
Adverse events.
Overall, 54 patients (90.0%) experienced an AE regardless of study drug causality (Table 3). AEs of special interest for treatment were proteinuria (n = 6), increased blood creatinine (n = 5), neurosensory deafness (n = 3; additional detail in the supplemental Data), conductive deafness and bilateral deafness (n = 2 each), increased ALT, increased aspartate aminotransferase, rectal hemorrhage, and decreased platelet count (n = 1 each).
AEs (≥5%) suspected related to either drug or combination therapy by the investigator were abdominal pain, increased UPCR (≥1.0 mg/mg; each 8.3%), and increased serum creatinine, diarrhea, and nausea (each 6.7%). Serious AEs irrespective of causality were reported in 17 patients (28.3%), of which 6 events in 4 patients were considered drug related (calcium deficiency, drug rash with eosinophilia and systemic symptoms [DRESS] syndrome, abdominal pain, dermoid cyst, and altered state of consciousness and pyrexia).
Five discontinuations due to AEs were reported: abdominal pain (n = 2), arthritis (n = 1), pruritus (n = 1), and suspected DRESS syndrome (n = 1). Further evaluation by independent experts did not reveal a consensus for the diagnosis of DRESS. Twenty-four hours after discontinuation, absolute eosinophil count and white blood cell count were normal at 0.1 × 109/L and 5.7 × 109/L in this patient.
No patient was reported to have cardiac failure or arrhythmia during the 2-year study. One patient died, attributed to a suspected central nervous system (CNS) infection. The patient had pyrexia with an altered state of consciousness suspected by the investigator to be related to infection and continuation of DFO treatment, although no autopsy was conducted and this could not be confirmed. The investigator stated that splenectomy and concomitant diabetes were contributory factors for the event. The majority of the AEs, including the patient death, were reported within the first 12 months of the study.
Laboratory parameters.
Mean ± SD creatinine clearance levels remained within the normal range (supplemental Figure 2). Seven patients had 2 consecutive decreases in creatinine clearance (CrCl) to category 60 to 90 mL per minute; 1 patient had 2 consecutive measurements below 60 mL per minute; no patients had CrCl levels <40 mL per minute. Two patients had 2 consecutive serum creatinine increases >33% above baseline and above the ULN, which were transient and resolved without intervention (n = 1) and managed with dose interruption/reduction (n = 1). Six patients (10.0%) had 2 consecutive measurements of UPCR >1.0 mg/mg, which either resolved without intervention (n = 3) or required dose interruption and/or reduction (n = 3).
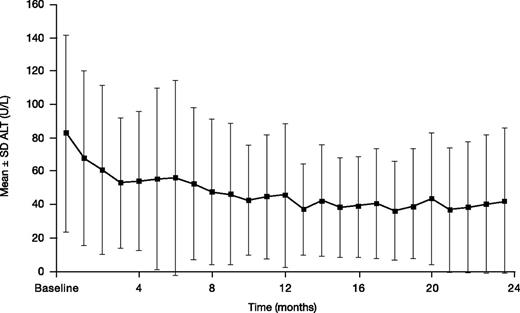
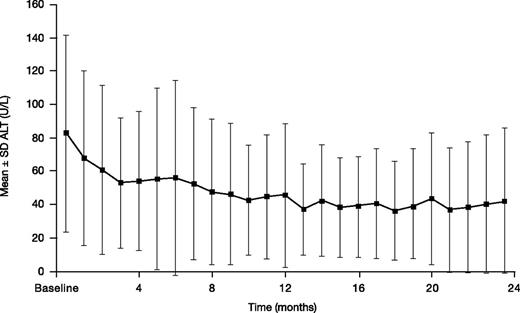
Mean ± SD ALT decreased from a baseline of 82.6 ± 59.1 to 42.2 ± 43.4 U/L at month 24 (n = 34; absolute change from baseline –33.0 ± 52.2 mg Fe/g dw; Figure 6). Two (3.3%) patients had 2 consecutive increases in ALT >5× ULN and >2× baseline.
ALT in patients treated with DFX-DFO in combination followed by DFX monotherapy over 24 months.
ALT in patients treated with DFX-DFO in combination followed by DFX monotherapy over 24 months.
Discussion
The objective of HYPERION was to evaluate the effectiveness of a DFX-DFO combination regimen in patients with severe myocardial siderosis, followed by DFX monotherapy in patients achieving mT2* ≥10 ms with a relative mT2* increase of ≥10% from baseline after 6 months. The underlying assumption behind the study design was that patients may benefit from combination therapy to rapidly remove iron when they are at risk of myocardial iron-related heart failure, but will adhere better over their lifelong chelation regimen to oral monotherapy once they have moved into a lower risk strata for heart failure (mT2* >10 ms).
The population enrolled in HYPERION had high baseline liver and myocardial iron, with mean LIC of 33.4 mg Fe/g dw and Gmean mT2* of 7.2 ms. Findings showed that combination DFX-DFO, followed by DFX monotherapy where appropriate, resulted in robust and clinically relevant improvements in mT2* at 12 (9% improvement) and 24 months (30% improvement); although examination of the 95% CIs of the month 12 and month 24 absolute change in mT2* may suggest statistical significance, this descriptive study was not powered for hypothesis testing. Importantly, a rapid and substantial decrease in LIC was also observed. A substantial proportion of patients (25.0%) also achieved sufficient control of mT2* to enable switch to DFX monotherapy after a limited duration of combination therapy. Although 7 of the 10 patients with baseline mT2* 5 to <6 ms discontinued, they may not all necessarily be considered treatment failures because reasons for discontinuation included relocation and inability to visit the investigational site. Furthermore, patients with mT2* 5 to <6 ms may be expected to have repeat measurements <5 ms due to the variability in CMR assessments at these values.
Myocardial iron removal with combination treatment was continuous, with an increasing proportion of patients who remained on the study achieving a clinically significant improvement in mT2* ≥10 ms and a ≥10% relative increase from baseline at each time point. When considering all patients initially enrolled, this increase was still evident, although less pronounced. The differences between the results of the post hoc ITT analysis and the prespecified analysis of patients remaining on treatment is accounted for by the relatively high rate of patient discontinuations, predominantly due to withdrawal of consent (n = 6) and loss to follow-up (n = 6). Improvements in MIC were observed after 12 months of treatment in patients remaining on the study, in contrast to LIC where the decrease was rapid, with substantial changes evident from month 6 onward. These trends toward greater improvement of mT2* and MIC over time may have been influenced by continued treatment and permitted DFX dose increases after month 6, highlighting the need for long-term treatment of myocardial iron removal, as well as dose adjustments based on both efficacy and safety parameters. These observations are also consistent with those of Noetzli et al using other chelation regimens, where improvement in mT2* generally followed improvements in LIC.27 Nevertheless, mT2* improvements were observed across the full range of baseline liver iron burdens, highlighting the effectiveness of this treatment modality in treating a wide range of patients. Although the improvement in mT2* was numerically greater in patients with baseline LIC <30 vs ≥30 mg Fe/g dw, there were insufficient patient numbers with low baseline LIC to systematically examine the influence of baseline LIC.
These findings need to be considered in the context of the heart, as one of the most sensitive organs to iron toxicity. Iron-related heart failure and death occur at tissue iron concentrations in the heart that are generally tolerated in the liver.25 Consequently, removal of a small amount of iron from the heart may have a greater impact on organ function compared with removal of an equivalent amount of liver iron. Furthermore, given the nonlinear relationship between mT2* and MIC,25 proportional improvements in mT2* from the low baseline levels in HYPERION relate to a larger degree of iron removal when compared with patients having higher baseline mT2*.
Importantly, while all patients had LVEF ≥56% at baseline, in patients remaining on treatment the mean LVEF remained stable throughout the study. There were also no new episodes of arrhythmias or cardiac failure reported, despite severe myocardial iron overload at baseline in all patients, imparting a high risk of heart failure within 12 months.1 Results highlight that appropriate chelation therapy can help manage this risk. Both high-dose DFO monotherapy and deferiprone-DFO combination therapy may have a role in treating thalassemia-major patients with heart failure (LVEF <56%)28 ; the efficacy of DFX-DFO combination therapy in such a patient population requires further investigation.
Advances in the management of cardiac complications have led to a longer life expectancy in patients with transfusion-dependent anemias,29,30 and other complications, including those related to liver iron overload, are increasingly being seen as a cause of death.31,32 Therefore, the rapid and clinically significant decrease in liver iron burden from severe levels at baseline in HYPERION is important. When compared with previous studies of DFX monotherapy, the mean ± SD absolute reduction in LIC after 1 year of DFX-DFO combination therapy was considerably higher despite similar levels at baseline: −14.3 ± 11.9 (HYPERION study) vs −8.9 ± 11.4 (CORDELIA study)10 and −6.6 ± 9.9 mg Fe/g dw (EPIC).5 Although more research is needed, this combination therapy regimen may be appropriate for a select group of patients who require a rapid decrease in liver iron even in the absence of myocardial siderosis, including circumstances such as pre-bone marrow transplantation, planned pregnancy, or when high doses of monotherapy are not well tolerated. Notably, LIC reduction was matched by an overall improvement in ALT levels, reflecting possible improvement in liver function in these patients. Substantial reduction in LIC may have also influenced the extent of mT2* improvement in some patients,27,33 particularly because the majority of patients had severe iron overload at baseline; this requires further study. Serum ferritin levels also decreased substantially, highlighting the reduction in total body iron stores with DFX-DFO treatment.
Although HYPERION was noncomparative and unblinded in nature, these results in a large patient population with severe total body iron burden expand on smaller pilot studies with DFX-DFO18,19,21 and provide insights into the combination treatment modality followed by less intensive monotherapy for a duration longer than 1 year. Tanner et al have also reported on combined chelation therapy in patients with both mild-to-moderate17 and severe15 myocardial siderosis but using deferiprone and DFO. The latter study assessed 15 patients with severe iron overload including myocardial siderosis (mean mT2* 5.7 ms and liver T2* 3.7 ms), and showed significant improvements in mT2* to 7.9 ms and in liver T2* to 10.8 ms at 1 year. LVEF increased significantly from 51.2% to 65.6%. A more recent study in patients with severe myocardial siderosis and heart failure showed significant improvements in mT2* and LVEF over time, but no differences between the DFO monotherapy and DFO-deferiprone combination arms.28 However, it is difficult to make comparisons between the studies given the differences in total body iron burden of the enrolled patients, as well as study design and clinical assessments. Furthermore, Tanner et al evaluated a combination regimen throughout the study durations, whereas HYPERION evaluated a treatment modality of combination followed by monotherapy and reported results together for those patients receiving combination therapy throughout and those who switched to monotherapy.
Safety findings were consistent with the established safety profile of DFX and DFO, with no unexpected findings.8-10,34 Renal and hepatic AEs in particular were not increased compared with previous reports of DFX monotherapy.10 Although the number of study discontinuations was higher than DFX monotherapy studies,5,6,10,35 particularly over the first 12 months, these discontinuations were largely due to logistical issues and not safety concerns. This is not unexpected because combination DFX-DFO therapy may be cumbersome, particularly while complying with the requirements of a clinical trial. Combination therapy should therefore be limited to as short a duration as possible, followed by monotherapy with deferasirox, which may be sufficient to control iron overload once patients have reached a lower-risk status for heart failure. Fifteen patients switched to monotherapy based on a favorable mT2* response (of whom 2 patients later returned to combination therapy). One of the difficult issues with cardiac management is whether to continue with combination therapy if severe body iron burden is reduced (eg, if LIC is <5 mg Fe/g dw or serum ferritin is <1000 ng/mL, but mT2* is still below 10 ms). Data on such patients are still limited. The only death in the study was due to a suspected, but not confirmed, CNS infection. Pyrexia with an altered state of consciousness in this patient was suspected by the investigator to be related to DFO treatment. Patients in this study received high doses of DFO with dose reductions as serum ferritin decreased. Although audiometric changes seen here were not suspected to be related to treatment, patients receiving DFO and DFX, in combination or as monotherapy, should receive regular audiometric monitoring.
In conclusion, DFX-DFO combination therapy followed by DFX monotherapy, in cases with sufficient myocardial T2* improvement, with appropriate dose adjustments, improved mT2* after 12 and 24 months of treatment. Furthermore, none of these high-risk patients who completed the study developed heart failure over 24 months. Importantly, there was a rapid and clinically meaningful reduction in liver iron in these patients with severe total body iron burden at baseline. The safety profile of both treatments in combination was clinically manageable with no additional safety concerns. Although further evaluation of DFX-DFO combination therapy in “real-world” clinical practice is desirable, it may be considered not only for the treatment of patients with severe transfusional myocardial siderosis requiring intensive chelation, but also in specific patients in whom a rapid reduction in liver iron is needed.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Debbi Gorman of Mudskipper Business Ltd for medical editorial assistance.
This work was supported by Novartis Pharma AG. Financial support for medical editorial assistance was provided by Novartis Pharmaceuticals. J.B.P. is supported by the NIHR University College London Hospitals Biomedical Research Centre (BRC).
The study was sponsored by Novartis Pharma AG and designed by the sponsor in close collaboration with the study steering committee. The sponsor conducted the statistical analysis. Authors had full access to the data and participated actively in interpreting data and writing and critically reviewing the article with the assistance of a medical writer funded by the sponsor.
Authorship
Contribution: Y.A., J.B.P., A.K., A.E.-B., M.E., Y.K., S.P., Z.K., V.V., and R.O. served as investigators on this trial, enrolled patients, contributed to data interpretation, and reviewed and provided their comments on this manuscript; Y.A., J.B.P., A.K., and M.D.C. served as Study Steering Committee members overseeing the conduct of the trial, from study design to analysis plan and data interpretation; D.H. and N.C. assisted in developing the trial protocol, coordinated the execution of the trial, and contributed to the analysis, interpretation, and reporting of the trial data; J.S. served as the trial statistician; Y.A. had final responsibility for the manuscript content and decision to submit for publication; and all authors approved the final manuscript.
Conflict-of-interest disclosure: Y.A. reports participating in a speaker’s bureau for, and receiving research grant support and honoraria from, Novartis Pharmaceuticals, and receiving research grant support from FerroKin BioSciences Inc. J.B.P. reports participation in advisory boards for Novartis Pharmaceuticals. A.K. reports receiving honoraria and research funding from Novartis Pharmaceuticals and participating in a speaker’s bureau. M.D.C. reports participating in Novartis Pharmaceuticals advisory boards. A.E.-B. reports participating in a speaker’s bureau for, and receiving grant funding and honoraria from, Novartis Pharmaceuticals and research grant funding and honoraria from Genzyme and ApoPharma Pharmaceuticals. R.O. received speaker’s honoraria from Novartis Pharmaceuticals. S.P. received research funding from Novartis Pharmaceuticals. Z.K. received research grants and speaker’s honoraria from Novartis Pharmaceuticals. V.V. received research grant support and lecture fees from Novartis Pharmaceuticals and research grant support from GPOLONE, Thailand, FerroKin Biosciences, and National Research University (NRU), Thailand. D.H., N.C., and J.S. are employees of Novartis Pharmaceuticals. The remaining authors declare no competing financial interests.
A complete list of the members of the HYPERION Investigators appears in the supplemental Data.
Correspondence: Yesim Aydinok, Ege University Hospital, Izmir, Turkey; e-mail: yesim.aydinok@ege.edu.tr.