Abstract
Acquired thrombotic thrombocytopenic purpura (TTP) is characterized by thrombocytopenia and microangiopathic hemolytic anemia (MAHA) without an obvious cause, and may include fever, mild renal failure, and neurologic deficits. It is characterized by a deficiency of the von Willebrand factor (VWF) cleaving enzyme, ADAMTS13 (a disintegrin and metalloproteinase, with a thrombospondin type 1 motif, member 13), resulting in formation of microthrombi in the high sheer environment of the microvasculature. This causes microvascular occlusion, MAHA, and organ ischemia. Diagnosis is based on the presence of clinical symptoms, laboratory aberrations consistent with MAHA, decreased ADAMTS13 activity, and possibly presence of anti-ADAMTS13 autoantibodies. Upfront treatment of acute TTP includes plasma exchange and corticosteroids. A significant number of patients are refractory to this treatment and will require further interventions. There are limited data and consensus on the management of the refractory TTP patient. Management involves simultaneously ruling out other causes of thrombocytopenia and MAHA, while also considering other treatments. In this article, we describe our management of the patient with refractory TTP, and discuss use of rituximab, increased plasma exchange, splenectomy, and immunosuppressive options, including cyclophosphamide, vincristine, and cyclosporine. We also review recent evidence for the potential roles of bortezomib and N-acetylcysteine, and explore new therapeutic approaches, including recombinant ADAMTS13 and anti-VWF therapy.
Case
A 25-year-old previously healthy woman presented with a 3-day history of fatigue, nausea, abdominal pain, and easy bruising. She was alert, oriented, afebrile, had mild abdominal tenderness, and purpura on her extremities. Her hemoglobin was 8.4 g/dL; platelets, 15 × 109/L; creatinine, 1.1 mg/dL; and her lactate dehydrogenase and reticulocytes were increased. Schistocytes and nucleated red blood cells were easily seen on her peripheral blood smear. In the absence of other obvious precipitators of thrombocytopenia and microangiopathic hemolytic anemia (MAHA), she was diagnosed with acquired thrombotic thrombocytopenic purpura (TTP). Blood samples were sent for ADAMTS13 (a disintegrin and metalloproteinase, with a thrombospondin type 1 motif, member 13) activity and inhibitor levels, and the patient was immediately started on plasma exchange (PEX) and prednisone 1 mg/kg per day. She responded well initially, and her platelets rose to 105 000/μL on day 4. However, on day 5, she was febrile and her platelets dropped to 40 000/μL.
Background
Acquired TTP was initially characterized by thrombocytopenia, MAHA, renal failure, neurologic deficits, and fever. However, it is now well accepted that neither renal failure nor high fevers are key diagnostic features. Thrombocytopenia and MAHA are required for diagnosis when TTP is suspected. TTP is a hematologic emergency, with a mortality of 90% if untreated. Treatment with PEX and corticosteroids significantly reduces the mortality to ∼10% to 15%.1,2
Acquired TTP is due to a deficiency of the von Willebrand factor (VWF) cleaving serine metalloprotease, called ADAMTS13.3,4 Anti-ADAMTS13 autoantibodies contribute to the pathogenesis of acquired TTP. This autoimmune process may be the result of: (1) neutralizing antibodies that inhibit ADAMTS13 proteolytic activity or (2) nonneutralizing antibodies that interfere with important ADAMTS13-binding partners or that enhance clearance of ADAMTS13.5-7 Congenital TTP is rare, and is due to an inherited deficiency of ADAMTS13.8 In the absence of ADAMTS13, uncleaved ultra-large VWF multimers bind to platelets in high sheer environments, forming inappropriate microthrombi that cause microvascular occlusion and MAHA, which results in organ ischemia.
Diagnosis of TTP
The initial diagnosis of TTP should be based on the clinical history, physical examination, routine laboratory investigations, and the definitive presence of schistocytes on the peripheral blood smear. Patients generally present with signs and symptoms, and have laboratory abnormalities that are reflective of the underlying microvascular thrombi, MAHA, and organ ischemia (Tables 1-29-12 ).9,13 The classic “pentad” of clinical features occurs only in 5% of patients, and may not be relevant to current practice now that the rapid initiation of PEX and evaluation of ADAMTS13 activity levels are widely done.10 A detailed evaluation should be performed to rule out other potential causes of thrombotic microangiopathy (TMA) (Table 3).
Clinical features, and signs and symptoms of TTP
| Clinical feature . | Signs/symptoms . |
|---|---|
| MAHA | Pallor, weakness, fatigue, jaundice |
| Thrombocytopenia | Petechiae and occasionally purpura |
| Bowel ischemia | Abdominal pain, nausea, vomiting, diarrhea |
| Cardiac ischemia | Chest pain, hypotension, heart failure |
| Central nervous system ischemia | Common: confusion, headache |
| Less common: coma, encephalopathy, stroke, seizure, focal abnormalities | |
| Renal ischemia | Hematuria, proteinuria |
| Fever | Fever (high fever with chills suspect other diagnosis) |
| Clinical feature . | Signs/symptoms . |
|---|---|
| MAHA | Pallor, weakness, fatigue, jaundice |
| Thrombocytopenia | Petechiae and occasionally purpura |
| Bowel ischemia | Abdominal pain, nausea, vomiting, diarrhea |
| Cardiac ischemia | Chest pain, hypotension, heart failure |
| Central nervous system ischemia | Common: confusion, headache |
| Less common: coma, encephalopathy, stroke, seizure, focal abnormalities | |
| Renal ischemia | Hematuria, proteinuria |
| Fever | Fever (high fever with chills suspect other diagnosis) |
Laboratory findings suggestive of acquired TTP
| Laboratory data . | Results . |
|---|---|
| Platelet count, ×103/μL | <30 |
| Hemoglobin, g/dL | <10 |
| Lactate dehydrogenase | Elevated |
| Haptoglobin | Decreased |
| Reticulocyte count | Increased |
| Indirect bilirubin | Increased |
| Peripheral blood smear | Increased schistocytes, nucleated red blood cells* |
| Creatinine | Mildly increased (<1.5 mg/dL) |
| Troponin T | May be increased |
| INR, PTT, fibrinogen | Normal |
| Laboratory data . | Results . |
|---|---|
| Platelet count, ×103/μL | <30 |
| Hemoglobin, g/dL | <10 |
| Lactate dehydrogenase | Elevated |
| Haptoglobin | Decreased |
| Reticulocyte count | Increased |
| Indirect bilirubin | Increased |
| Peripheral blood smear | Increased schistocytes, nucleated red blood cells* |
| Creatinine | Mildly increased (<1.5 mg/dL) |
| Troponin T | May be increased |
| INR, PTT, fibrinogen | Normal |
INR, international normalized ratio; PTT, partial thromboplastin time.
Consider a bone marrow biopsy in the presence of numerous nucleated red blood cells to rule out an alternative etiology, for example, occult systemic malignancy.9-12
Differential diagnosis of thrombocytopenia and MAHA
| Major disorder . | Examples . |
|---|---|
| Autoimmune disorders | SLE, scleroderma, antiphospholipid antibody syndrome |
| Systemic infection | Viral (CMV, adenovirus, HSV), bacterial (meningococcus, pneumococcus), fungal (Aspergillus) |
| Systemic malignancy | DIC or tumor emboli syndrome |
| Vasculitis | |
| DIC | Sepsis, cancer |
| Pregnancy associated | HELLP, eclampsia, HUS |
| Drugs | Quinine, simvastatin, interferon, calcineurin inhibitors |
| Malignant hypertension | |
| HUS |
| Major disorder . | Examples . |
|---|---|
| Autoimmune disorders | SLE, scleroderma, antiphospholipid antibody syndrome |
| Systemic infection | Viral (CMV, adenovirus, HSV), bacterial (meningococcus, pneumococcus), fungal (Aspergillus) |
| Systemic malignancy | DIC or tumor emboli syndrome |
| Vasculitis | |
| DIC | Sepsis, cancer |
| Pregnancy associated | HELLP, eclampsia, HUS |
| Drugs | Quinine, simvastatin, interferon, calcineurin inhibitors |
| Malignant hypertension | |
| HUS |
CMV, cytomegalovirus; DIC, disseminated intravascular coagulation; HELLP, hemolysis, elevated liver enzymes, low platelets; HSV, herpes simplex virus; HUS, hemolytic uremic syndrome; SLE, systemic lupus erythematosus.
A low baseline ADAMTS13 activity of <10%, with or without the presence of an anti-ADAMTS13 autoantibody, in a patient with thrombocytopenia and MAHA, strongly supports the diagnosis of TTP.13-16 Samples for ADAMTS13 analysis should be taken prior to treatment to avoid erroneous results and diagnostic confusion. However, 78% of patients continue to have pre-PEX ADAMTS13 activity of <10%, even after 3 days of PEX.17 ADAMTS13 levels between 10% and 40% have been reported in acquired TTP and other conditions, including pregnancy, uremia, and inflammation.18,19 The ADAMTS13 activity assays are neither 100% sensitive nor specific, and the results are method dependent and may not be immediately available.20 Therefore, the decision to start or continue treatment with PEX must be a clinical decision based on history, physical examination, and laboratory tests. The practitioner should not wait for results of the ADAMTS13 assay before starting PEX.
Treatment of acquired TTP
PEX and corticosteroids are the mainstays of treatment of acquired TTP. Individuals with thrombocytopenia and MAHA, and without secondary causes of TMA, meet the working diagnosis of acquired TTP. These patients should immediately receive PEX with at least 1 plasma volume (PV), and the treatment continued daily until a response is achieved. According to the 2012 American Society of Apheresis Consensus Conference on TTP, response is defined as achieving a platelet count >150 000/μL for 2 consecutive days, a normal or near normal lactate dehydrogenase (LDH), and stable or improving neurologic deficits.21 The number of PEX treatments needed is variable, and may be higher in TTP patients with high titer anti-ADAMTS13 autoantibodies.15 We advocate supplementing PEX therapy with corticosteroids immediately upon diagnosis. PEX is superior to plasma infusion in TTP, and is associated with decreased mortality.1 Although frequently intolerable, very large doses (25-30 mL/kg) of plasma infusion might be sufficient until PEX can be initiated.22 PEX is thought to work by replacing the deficient ADAMTS13 and removing inhibitory autoantibodies.23 In patients with severe disease, or progressive symptoms over the first few days, PEX may be intensified to 1.5 PV and reduced to 1 PV once the clinical situation and laboratory findings stabilize.1,24 Once a response has been achieved, PEX can be stopped without a taper while closely monitoring blood counts and LDH. Limited evidence suggests that there is no value to tapering the frequency and/or volume of PEX.25 The apheresis catheter should be removed as soon as the patient has stabilized off apheresis, or at the first sign of infection.
Refractory TTP
The reported incidence of patients who do not respond to PEX and corticosteroids and require additional therapy varies between 10% and 42%.26-29 In much of that literature, refractory TTP is defined as a failure of platelet response after 4 to 7 days of PEX, or a clinical deterioration in a patient receiving standard therapy. In the case of possible refractory acquired TTP, it is absolutely imperative to reevaluate the clinical scenario in order to identify other causes of thrombocytopenia and MAHA that may require additional therapy. It is in this situation that the ADAMT13 activity and inhibitor analysis is perhaps most helpful. Patients who have very low ADAMTS13 levels and high inhibitor titers most likely have TTP, and should be treated with therapies that will be described in this review. Patients who do not have low ADAMTS13 levels might not actually have TTP, and should be extensively evaluated for other causes of thrombocytopenia and MAHA.
For example, patients who initially respond to PEX and then deteriorate should be evaluated for sepsis due to a line-associated infection or for drug-induced thrombocytopenia caused by a recently initiated medication, such as an antibiotic. If refractory TTP still remains the diagnosis after clinical reevaluation and confirmation of TTP with low ADAMTS13 levels, immunosuppressive agents may be considered. There is limited information, and no randomized controlled trials on the use of immunosuppressive agents in acquired TTP. Even less information or consensus is available on the management of refractory acquired TTP.
Treatment options for TTP refractory to PEX
Corticosteroids
Corticosteroids are used in the acute management of acquired TTP, and should be started upfront together with PEX.2,30,31 Steroids are believed to suppress the production of anti-ADAMTS13 autoantibodies. However, the basis for this knowledge has not been demonstrated in clinical trials. For the initial treatment of acquired TTP, we start prednisone 1 mg/kg per day in all patients. However, for acutely ill refractory patients, who are clinically unstable or who have neurologic symptoms, we consider increasing the immunosuppression. A randomized trial of standard dose (1 mg/kg per day) vs a higher dose (10 mg/kg per day for 3 days) IV methylprednisolone revealed significantly improved remission rates for the latter.31 In patients with clinical deterioration despite standard doses of corticosteroids, we consider the administration of high-dose methylprednisolone 1 g per day for 3 days.13,21,31,32
Twice-daily PEX
Twice-daily PEX is a treatment option in refractory acquired TTP, albeit with limited data on its effectiveness. Twice-daily PEX is sometimes initiated when an acutely ill patient, who initially responded to single-volume-daily PEX, has a sudden decline in platelet count or develops new neurologic symptoms.24 In a retrospective review of the Oklahoma registry, only 3 of 28 patients who received twice-daily PEX appeared to obtain any benefit.24 Like many retrospective reviews, a clear benefit is not definitive because the initiation or intensification of other treatments is often done concurrently. We do not routinely use twice-daily PEX when managing our refractory patients. Cryosupernatant (sometimes called cryopoor plasma), which is relatively deficient in VWF multimers, appears to be equally as effective as fresh-frozen plasma in the management of TTP.33 For this reason, we typically use standard fresh-frozen plasma as the replacement fluid during apheresis of these patients.
Rituximab
Rituximab, a monoclonal antibody targeting the CD20 antigen present on B lymphocytes, is often used in treating refractory or relapsed TTP with good response rates.34-37 Most of the data are based on case reports and case series where rituximab was used concomitantly with other immunosuppressive agents.23,34,36-42 A recent thoughtful review on this topic adds some clarity to its use in this clinical situation.43 In several case series, treatment of acute refractory and/or relapsing TTP with rituximab alone resulted in clinical remission in 87% to 100% of patients, and platelet recovery within a median of 11 to 14 days after the first dose.34,36,40 An observational study comparing 21 refractory TTP patients treated with rituximab and historical controls revealed a 100% response rate and decreased time to platelet recovery in the patients receiving rituximab as compared with a 78% response rate in the controls.44 Anti-ADAMTS13 antibodies disappear, and ADAMTS13 activity levels significantly improve after the administration of rituximab.34,36 In the absence of a randomized prospective trial, it is difficult to be certain, but the currently available data suggest that rituximab may increase ADAMTS13 activity, decrease the time to platelet count response, reduce the duration of PEX, and potentially be effective in preventing relapses. In our opinion, it is now reasonable to consider treating individuals who have refractory acquired TTP with rituximab, if they have failed to respond to PEX and steroids.
Several questions still remain about the optimal dosing, timing of infusions, role of maintenance therapy, and long-term side effects of rituximab. The most frequently used dose is 375 mg/m2 once weekly for 4 weeks. This dose is based on what is used to treat lymphomas. Various groups have tried other dosing regimens for TTP, including giving rituximab 375 mg/m2 on days 0, 3, 7, and 14.44 Peripheral CD20+ B-cell depletion occurs after a median of 3 days (range, 1-14 days).45 However, the effect of rituximab on B-cell clearance in lymph nodes, tissues, and the spleen is unknown. Following the use of lower-dose rituximab in autoimmune disease, a pilot study to evaluate rituximab (100 mg per week for 4 doses) as an adjunct to PEX and corticosteroids in TTP is currently under way (NCT01554514, http://clinicaltrials.gov). Because 65% of rituximab is removed during PEX, it may be more effective to give rituximab more frequently than once a week.45 However, further study is needed to determine the optimal dose and scheduling of rituximab and PEX. Rituximab has generally been well tolerated, but it can produce infusional reactions. Rare severe complications, including progressive multifocal leukoencephalopathy, herpes zoster transverse myelitis, encephalitis, paraplegia, hepatitis B reactivation, and cardiac toxicity have been reported in patients treated with rituximab for any indication. However, most of these complications have not been reported in TTP patients treated with rituximab.42
In our current practice, we consider treating patients who have acute refractory acquired TTP with rituximab when there has been no platelet response after 4 to 7 days on PEX or when there is clinical deterioration while on PEX and corticosteroids. We give a dose of 375 mg/m2 once a week for 4 weeks, and we try to time the daily PEX treatments so that the PEX following each rituximab infusion occurs ∼24 hours after the administration of rituximab.
Treatment options after PEX, steroids, and rituximab do not work
For patients who are still refractory and fail to respond to increased doses of corticosteroids, larger volume PEX, and rituximab, alternative therapies to consider are splenectomy or stronger immunosuppression, such as cyclophosphamide, vincristine, and cyclosporine. These modalities have been used in clinical practice for many years, with primary evidence derived primarily from small case series and anecdotal evidence.
Splenectomy
The response rate of refractory TTP to splenectomy has been variable in the limited published literature. In a case series of 6 patients with refractory TTP undergoing splenectomy, 1 patient died immediately postsplenectomy, whereas 5 had complicated clinical courses, including worsening hematocrit, thrombocytopenia, and even coma.2 On the other hand, in a different case series of 6 patients with refractory TTP, splenectomy induced remission within 6 days or less in every patient.46 The 10-year relapse-free survival rate for splenectomy in refractory/relapsed TTP is 70%.47 A literature review described 74 cases of refractory TTP, and found that only 8% of the patients failed to respond to splenectomy.48 Splenectomy presumably removes not only a major site of anti-ADAMTS13 antibody production, but it can also remove a major site of clearance of opsonized ADAMTS13. When considering splenectomy, the initial risks of the surgical procedure, and the long-term risk of infections, need to be weighed against the potential benefits in the clinical scenario. With newer modalities of immunosuppression showing potential promise in the management of refractory TTP, splenectomy may be a less favorable option in the future. At our Institution, we only rarely consider splenectomy in stable patients who are refractory to rituximab.
Cyclosporine
Cyclosporine is an immunomodulating drug that inhibits T-cell activation, thereby inhibiting interleukin-2 receptor expression and interleukin-2 production. Cyclosporine has been successfully used to treat refractory TTP in case reports and in a cohort study.49-53 In a study evaluating the efficacy of PEX and cyclosporine in the treatment of TTP (included refractory TTP cases), remission was achieved in 89% of all patients.53 Although this study also showed improvement in ADAMTS13 activity and in the suppression of ADAMTS13 inhibitors, the response rates were closely similar to those achieved with PEX alone. In the treatment of TTP, cyclosporine is generally continued for at least 6 months, and in the few cases of relapse, patients have responded when retreated with cyclosporine. Cyclosporine is rarely used because it can also cause a TMA independent of TTP. However, we believe that in patients who have stable renal function, the addition of cyclosporine is a reasonable option for treating TTP that is refractory to PEX, steroids, and rituximab.
Cyclophosphamide and vincristine
Prior to the availability of rituximab, immunosuppressive therapy with cyclophosphamide was considered a reasonable upfront option for treating patients refractory to PEX and steroids. This was based on the results of a few case reports and case series.54,55 More recently, it has been used in patients who are refractory to rituximab, or used in combination with rituximab for TTP patients who are refractory to steroids and PEX.56-58 In a recent case series of 5 patients with refractory TTP (refractory to PEX, steroids, along with vincristine or rituximab or both), who were then treated with 3 to 6 pulses of cyclophosphamide (500-750 mg/m2 per pulse), a durable platelet recovery was seen at a median period of 10 days after the first cyclophosphamide pulse.56 Side effects of cyclophosphamide include bone marrow suppression, infection risk, infertility, and a potential long-term risk of myelodysplastic syndrome/leukemia. The additional risk of acute infection with the possibility of an exacerbation of TTP makes this a less favorable option. Therefore, now that rituximab is widely used, we only consider cyclophosphamide as a potential salvage therapy in rituximab refractory TTP. In addition, we tend to avoid treating young individuals with cyclophosphamide, due to the risk of infertility or malignancy.
Vincristine has also been used in relapsed or refractory TTP with response rates of 50% to 87%.59-61 The side-effect profile includes mild peripheral paresthesias, muscle weakness, paralytic ileus, leukopenia, and transient alopecia.62 Vincristine may work better when used in the initial treatment of patients with TTP, rather than for treating refractory patients.60,62 A combination of cyclophosphamide and vincristine has also been used successfully in treating refractory TTP.63,64 In the era of rituximab, we would consider this combination only in those patients who are refractory to rituximab.
Emerging roles for other agents
In the last few years, new potential therapies for refractory TTP have shown promise, including bortezomib and N-acetylcysteine (NAC). However, limited data on these treatment options are available, and further study is required.
Bortezomib
Recently, the proteasome inhibitor bortezomib has been shown in several case reports to induce remission and deplete ADAMTS13 autoantibodies in refractory and relapsing TTP.65-68 Shortt et al reported on a case of a woman with TTP who was refractory to PEX, prednisone, cyclophosphamide, rituximab, and NAC, despite having documented depletion of B cells within her bone marrow.65 Because this patient still had plasma cells that could have been synthesizing ADAMTS13 autoantibodies, she was treated with bortezomib with a good outcome. A similar rationale has driven the use of bortezomib to deplete the alloreactive HLA-matched antibodies encountered during solid-organ transplant rejection.69 The few case reports describing the use of this therapy in patients with refractory TTP have used a dosing regimen that is typical for the treatment of multiple myeloma, in which bortezomib is given at 1.3 mg/m2 on days 1, 4, 8, 11, and repeated every 21 days. Further studies to establish the role of bortezomib in the management of refractory and/or relapsing TTP are needed.
N-acetylcysteine
NAC is a widely available and affordable drug used in the treatment of acetaminophen overdose, or to decrease the viscosity of mucous secretions in respiratory disorders. It has recently been tested as a potential adjunct to PEX in the treatment of TTP. NAC has a free sulfhydryl group that reduces the disulfide bonds in mucin polymers, thereby decreasing the size and viscosity of mucins.70 VWF, like mucin, is also a polymer of dimers linked by disulfide bonds. Based on the observation that mucin and VWF share structural similarities, Chen and colleagues hypothesized that NAC could substitute for ADAMTS13 and reduce the size of ultra-large-molecular-weight VWF (ULVWF).71 They were able to show that in vitro, NAC reduces soluble VWF multimers, decreases the size of VWF multimers, and rapidly degrades ULVWF multimers. NAC also inhibits VWF-dependent platelet agglutination and adhesion to collagen. NAC reduced VWF multimer size in wild-type mice. In ADAMTS13-deficient mice, NAC reduced the formation of thrombi in mesenteric venules. NAC was recently evaluated as adjunctive TTP therapy, and was used to manage a PEX and corticosteroid-treated patient who had persistent thrombocytopenia, neurologic, and cardiac symptoms.70,72 Despite the initiation of rituximab, the patient continued to deteriorate with coma, low blood pressure, and continued fever. NAC was initiated at 150 mg/kg IV over 1 hour after PEX, and daily NAC infusions were continued for 10 days at 150 mg/kg given over 17 hours after PEX. The patient came out of the coma 18 hours after starting NAC. The literature also describes 2 additional patients with refractory TTP who failed to respond to NAC therapy.65,73 The discrepancies between these outcomes might be attributable to the different NAC doses used in the case reports.74 At this point, NAC represents a theoretically appealing drug for TTP therapy. However, there is very little information at this time to state that NAC has any clinical value in the treatment of TTP. A pilot study to evaluate the use of NAC in patients with TTP and its effect on VWF function and tolerability is currently recruiting patients (NCT01808521, http://clinicaltrials.gov). To further understand the potential role of NAC in the management of TTP, enrollment in clinical trials is encouraged.
Eculizumab
The anti-C5 monoclonal antibody (mAb), eculizumab, has been successfully used to treat complement-mediated TMA.75 Recently, a patient with MAHA (who was refractory to PEX, steroids, vincristine, rituximab, and NAC) with heavy skin complement deposition responded to eculizumab.73 However, this patient had a complement factor H gene polymorphism and anti-complement factor H antibodies. This demonstrated that the diagnosis was actually a complement-mediated TMA instead of TTP.76 To date, there is no evidence that eculizumab should be used to treat acquired TTP.
Emerging therapies for TTP
Recombinant ADAMTS13
Plasma exchange and immunosuppression for the treatment of refractory idiopathic TTP patients have their limitations and associated side effects. Therefore, novel targeted approaches that capitalize on our understanding of the underlying pathophysiology are needed. The characterization, cloning, and expression of recombinant ADAMTS13 (rADAMTS13) has enabled studies of replacement therapy.77 In vitro replacement of rADAMTS13 has successfully replenished VWF cleaving activity in the plasma from congenital and acquired TTP patients.77,78 Recently, Schiviz et al generated a murine model of TTP by infusing ADAMTS13 knockout mice with recombinant human VWF containing ULVWF multimers.79 Interestingly, prophylactic administration of recombinant human ADAMTS13 protects these mice from developing TTP. Thus, recombinant ADAMTS13 might be a potential and promising therapy for humans with congenital TTP.
A potential problem in treating acquired TTP with rADAMTS13 is that the anti-ADAMTS13 autoantibodies could bind to and accelerate the clearance of infused rADAMTS13. Giving large doses of rADAMTS13 might overcome this problem.80 A “designer” rADAMTS13 mutant has been generated to contain substitutions of the key antigenic residues that are frequently targeted by inhibitory antibodies.81 In theory, the administration of such a mutant ADAMTS13 would be resistant to the patient’s inhibitory autoantibodies, but still be able to degrade ULVWF multimers, and thereby arrest the disease. Further study is required to determine whether this concept is correct.
Anti-VWF therapy
A key feature of TTP is the formation of platelet-VWF–rich thrombi in the microvasculature. These microthrombi form due to the A1 domain of VWF binding to the platelet GpIbα receptor.82 Blocking the A1 domain could impair the binding of VWF to platelets, and prevent microthrombi formation in patients with TTP. Thus far, 3 different strategies have been used to block the VWF A1 domain.82 An aptamer (ARC1779), a humanized mAb (GBR600), and a bivalent single-chained antibody (ALX-0681) all bind to the VWF A1 domain and impair VWF binding to platelets in vitro.83-85 The aptamer, ARC1779, has been the most studied of the anti-VWF agents, and preliminary studies have shown improvement in the platelet count and LDH in patients with acquired TTP.86,87 In a double-blind placebo-controlled trial that closed prematurely, ARC1779 together with PEX suppressed VWF activity, and was well tolerated.84 However, it did not appear to provide benefit in preventing the formation of VWF-mediated microthrombi. GBR600 is an inhibitory anti-VWF mAb, which induced a rapid recovery of severe thrombocytopenia in baboons, and appears promising in preliminary studies.85 ALX-0681 is a single-chained antibody (a so-called Nanobody) that targets the A1 domain of VWF, and effectively prevents the onset of severe thrombocytopenia and hemolytic anemia in a baboon model of acquired TTP.83 It is notable that this Nanobody did not cause excessive bleeding. These new agents that target VWF represent exciting and novel approaches in the treatment of acquired TTP. Although these therapies will likely not replace PEX, they might prove to be useful adjuvants.
Case
Was the platelet drop due to refractory TTP or another cause of thrombocytopenia? Blood cultures were positive for coagulase-negative Staphylococcus. She was started on antibiotics, the catheter was exchanged, and daily PEX continued, with normalization of her platelet count 12 days after presentation.
Summary
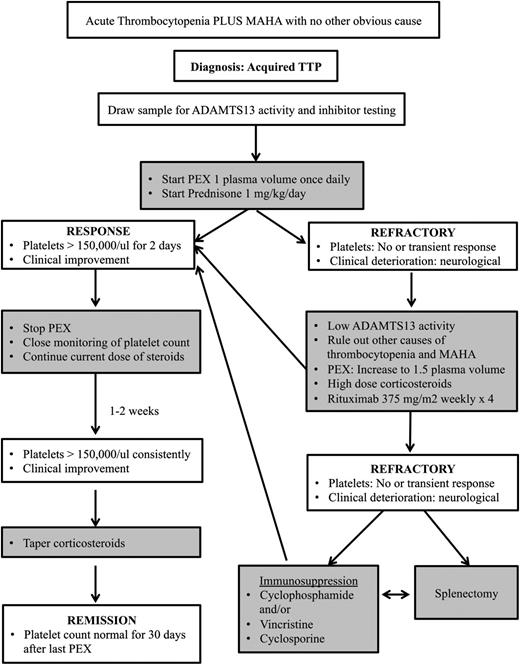
In summary, in a patient with acquired TTP that is refractory to PEX and corticosteroids, it is important to ensure that other causes of thrombocytopenia and MAHA have been ruled out, including sepsis and medication effects (Figure 1). In patients with an acute deterioration, we will consider administering methylprednisolone 1 g daily for 3 days. We will also increase the PEX volume to 1.5 PV. If the patient does not respond or continues to deteriorate, we will start rituximab at 375 mg/m2 weekly for 4 weeks. In those patients who are refractory to rituximab, other options for immunosuppression that we may consider include cyclosporine, cyclophosphamide, or vincristine. The choice of drug is based upon several factors, including the patient’s age, other comorbidities (including renal failure), drug access, and the physician’s comfort level and experience. In the stable patient who is refractory to rituximab, we may also consider splenectomy. There is currently not enough information on bortezomib and NAC to incorporate them into our standard protocols for the management of refractory TTP. Preliminary studies on rADAMTS13 and anti-VWF therapy as potential adjuvant therapies show promise, but it is too soon to say whether they will prove to be useful agents.
Acknowledgments
The authors are grateful to Dr James George for his thoughtful comments on this manuscript. This work was supported by the National Institutes of Health, National Heart, Lung, and Blood Institute grants HL120846, HL40387, HL097064, and HL083392.
Authorship
Contribution: F.A.S. and C.S.A. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Farzana A. Sayani, Perelman School of Medicine at the University of Pennsylvania, 3rd Floor Dulles Building, 3400 Spruce St, Philadelphia, PA 19104; e-mail: farzana.sayani@uphs.upenn.edu; or Charles S. Abrams, Perelman School of Medicine at the University of Pennsylvania, 421 Curie Blvd, Biomedical Research Building II/III #812, Philadelphia, PA 19104; e-mail: abrams@upenn.edu.