In this issue of Blood, Buchanan and coauthors present the development of a specific antidote for ticagrelor.1 In fact, this is the first specific antidote against any antiplatelet agent.
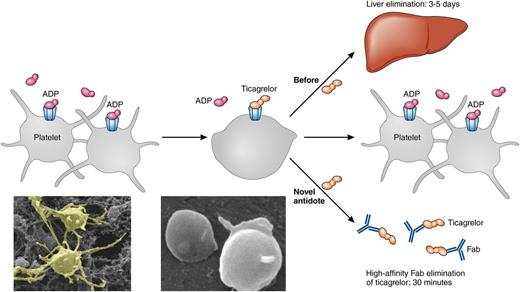
The figure illustrates platelets activated by ADP that aggregate. After treatment with the ADP antagonist ticagrelor, aggregation is limited. If bleeding occurs or the patient needs to undergo urgent surgery, elimination of ticagrelor is currently dependent on liver elimination requiring 3 to 5 days. With the high-affinity Fab ticagrelor antidote, the elimination can be achieved within 30 minutes. After elimination of ticagrelor, the platelets can aggregate in response to ADP again and bleeding can be mitigated. Original magnification ×10 400. Professional illustration by Patrick Lane, ScEYEnce Studios.
The figure illustrates platelets activated by ADP that aggregate. After treatment with the ADP antagonist ticagrelor, aggregation is limited. If bleeding occurs or the patient needs to undergo urgent surgery, elimination of ticagrelor is currently dependent on liver elimination requiring 3 to 5 days. With the high-affinity Fab ticagrelor antidote, the elimination can be achieved within 30 minutes. After elimination of ticagrelor, the platelets can aggregate in response to ADP again and bleeding can be mitigated. Original magnification ×10 400. Professional illustration by Patrick Lane, ScEYEnce Studios.
Ticagrelor is a widely used reversible adenosine 5′-diphosphate (ADP) P2Y12 antagonist for treatment of patients with acute coronary syndrome (ACS). In the Platelet Inhibition and Patient Outcomes trial, ticagrelor reduced the combined end point of death from vascular causes, myocardial infarction, or stroke for up to 12 months, and even reduced total mortality.2 However, patients also experienced increased bleeding events not associated with coronary-artery bypass grafting (CABG). In the recently published Prevention of Cardiovascular Events in Patients with Prior Heart Attack Using Ticagrelor Compared to Placebo on a Background of Aspirin–Thrombolysis in Myocardial Infarction 54 trial, patients with a myocardial infarction 1 to 3 years prior to enrollment were randomized to 2 different doses of ticagrelor vs placebo.3 Even though the study reached its primary end point and demonstrated a significant reduction in ischemic events, this reduction was matched by a similar number of bleeding complications. Furthermore, a patient on ticagrelor cannot undergo urgent CABG or other surgical procedures without increased bleeding risk unless treatment with ticagrelor is interrupted for 3 to 5 days, which increases the risks and causes discomfort for the patient as well as a prolonged hospital stay and higher health care costs.
The development of new antithrombotic medications may have reached its end due to an inevitable increase in bleeding complications when new agents are added: the addition of new antithrombotic drugs on top of already established guideline-directed therapies now often results in a neutral balance between ischemic end points and bleeding complications.4,5 Bleeding is associated with increased long-term mortality.6 The explanation for this is complex, with contributing factors including increased anemia, blood transfusions, inflammation, surgery, and complications due to prolonged hospital care. For bleeding caused by antiplatelet agents, transfusion of platelets may be of some benefit, but specific treatment is not available.
The antidote against ticagrelor developed by Buchanan et al is a human antibody antigen-binding fragment (Fab) with a high affinity (20 pM) for both ticagrelor and its active metabolite without binding to ADP or other purines. The design of the Fab was improved by crystallographic determination of the Fab-ticagrelor complex. The markedly higher affinity of the antidote (pM) compared to that of ticagrelor for the P2Y12 receptor (nM) results in elimination of both circulating and P2Y12-receptor–bound ticagrelor. In the study, the antidote reversed the antiplatelet effect of ticagrelor in human platelets in vitro, and rapidly restored ADP-induced aggregation in vivo in mice. Furthermore, the antidote normalized ticagrelor-dependent bleeding in a mouse model of acute surgery. The neutralizing effect had a rapid onset within 30 minutes (see figure).
A specific antidote for ticagrelor will be of great value in 2 clinical situations. First, when a patient suffers a life-threatening bleeding complication, the rapid neutralization of circulating ticagrelor may limit the extent of the bleeding, stabilizing the patient and limiting the long-term risk after a bleeding event. Second, a patient acutely treated with ticagrelor for an ACS who needs urgent CABG could undergo surgery within an hour instead of waiting several days. Similarly, a patient on maintenance therapy may undergo noncardiac emergency surgery with limited bleeding risk.
This is the first development of a specific antidote for an antiplatelet agent, fulfilling a long-sought need. For decades, we have used aspirin, clopidogrel, prasugrel, dipyridamol, abciximab, and cilostazol, where the only option for reversal was waiting for systemic elimination of the agent or platelet regeneration, which takes 5 to 10 days. Together with similar developments for newer orally administered anticoagulants such as the antidote idarucizumab for dabigatran,7,8 this represents a new era for antithrombotic drug development. It is possible that regulatory demands will increase for a simultaneous development of antidotes before approving antithrombotic drugs. In fact, a phase III study with available antidote may tip the net balance between anti-ischemic effect and the long-term effects of bleeding complications in a favorable way. It could also be a clinical advantage for newer antiplatelet agents compared to older antiplatelet medications that lack antidotes.
The development of a highly specific, rapid, and potent antidote for ticagrelor is an important achievement with great potential to improve patient safety. The findings in human platelets in vitro and in mice models in vivo are promising. However, clinical studies are needed to confirm the effects in humans.
Conflict-of-interest disclosure: D.E. has received speaker’s honoraria from AstraZeneca, Eli Lilly, and Boehringer Ingelheim.