Abstract
Over the last few decades, significant improvement in outcomes has been observed for myeloma patients, mainly as a result of the use of currently available approved antimyeloma agents, along with combining autologous stem cell transplantation in the treatment of myeloma. With more targeted agents in development, the treatment of a myeloma patient at relapse has become complicated and, as a consequence, results in vast heterogeneity in treatment patterns. Although a consensus on the timing of initiation of treatment, the choice of agents to be used, and the role of transplant is less clear, we describe an evidence-based approach and the factors to consider upon relapse. We describe additional newer agents and targets that are under development, with the goal of achievement of durable remissions for myeloma patients.
Introduction
Treatment choices for myeloma patients at each phase of their disease has become a complex decision-making process, in large part because of the deeper understanding of plasma cell biology, which resulted in more therapeutic options at each stage of treatment. Treatment of relapsed/refractory multiple myeloma (RRMM) presents a special therapeutic challenge, because of the heterogeneity of disease at relapse and the absence of clear biological-based recommendations regarding the choice of salvage therapies at various time points of disease progression. With increasing recognition of the inherent clonal heterogeneity and genomic instability of the plasma cells influencing both inherent and acquired therapeutic resistance, the identification of the optimal choice and sequence of therapies has become critical. Positively, several new agents and targets are currently under development and show considerable promise. The next-generation proteasome inhibitors (PIs) (ixazomib, marizomib, and oprozomib), other molecularly-targeted therapies directed at specific cell-signaling pathways (including histone deacetylase inhibitors, PI3K/AKT/mTOR inhibitors, Hsp90 inhibitors, cell-cycle inhibitors, kinesin spindle-protein inhibitors) are currently in development. Even newer approaches such as monoclonal antibodies targeting SLAMF7, CD38, CD138, and others have also demonstrated promising antimyeloma activity. This review focuses on evaluating various available treatment choices and options of treatment strategies at relapse, describes the rationality of combinations with established and newer agents, and highlights the emerging drugs currently under investigation and in various stages of clinical development.
Definitions
To enable formulation of a plan of treatment at relapse, it is important to delineate definitions of relapse and the optimal timing to initiate treatment. According to the International Myeloma Working Group criteria, progressive disease (PD) is defined by at least a 25% increase from nadir in the serum paraprotein (absolute increase must be ≥0.5 g/dL) or urine paraprotein (absolute increase must be ≥200mg/24 hours), or in the difference between involved and uninvolved serum-free light-chain (FLC) levels (with an abnormal FLC ratio and FLC difference >100 mg/L). In patients who lack measurable paraprotein levels (oligo- or nonsecretory myeloma), an increase in bone marrow plasma cells (≥10% increase) or new bone/soft tissue lesions increasing the size of existing lesions or unexplained serum calcium >11.5 mg/dL is used to define disease progression.1 “Relapsed and refractory” myeloma is defined as progression of therapy in patients who achieve minor response (MR) or better, or who progress within 60 days of their last therapy. Patients who never achieve at least a MR to initial induction therapy and progress while on therapy are defined as “primary refractory.” Relapsed myeloma is defined as disease in a myeloma patient who has previously been treated and has evidence of PD as defined here before, and who at the time of relapse does not meet the criteria for relapsed and refractory or primary refractory myeloma.2
Treatment options for relapsed and refractory myeloma
Various options exist currently for treatment of RRMM. In addition to the approved newer myeloma agents (immunomodulatory agents [IMiDs] and PIs), combinations of cytotoxic agents and high-dose therapy with autologous stem cell transplant (HDT-SCT) have led to significant survival improvements seen currently.
Approved myeloma agents
IMIDs.
Thalidomide.
Thalidomide (THAL), the prototype IMiD, inhibits angiogenesis and induces apoptosis of established neovasculature. With the addition of dexamethasone (DEX) (TD) the overall responses rates (ORRs) were augmented to 55% compared with the ORR of 24% with THAL alone.3,4 The current role of THAL in the management of RRMM is probably restricted, largely owing to the preceding use of next-generation IMiDs and the lack of data on the efficacy of THAL in lenalidomide (LEN)- or pomolidomide (POM)-refractory patients. Furthermore, neurotoxicity is considerable, and this risk needs to be weighed against the availability of newer and more effective options. However, THAL is safe in patients with renal failure and can combine well with PIs (bortezomib [BTZ]5,6 and carfilzomib [CFZ]7,8 ) and non-nephrotoxic chemotherapies. It is also not myelosuppressive and may have a role in patients with severe cytopenias and as a part of regimens such as TD+cyclophosphamide9,10 or melphalan,11 which can be considered as palliative in patients with no other options. THAL has been combined with pegylated doxorubicin (PLD)12 in patients with relapsed myeloma achieving median progression free survival (PFS) of 21 months. Combination regimens of THAL with acceptable response rates are illustrated in Table 1. In the refractory setting, it can be combined with other agents such as vorinostat, BTZ, or CFZ, achieving an overall clinical benefit rate as high as 72.5%, even among high-risk patients.13
Lenalidomide.
LEN is an analog of THAL with higher potency and less toxicity.14 Two parallel randomized phase 3 trials (MM-00915 and MM-01016 ) comparing LEN in combination with DEX (RD) to DEX alone among RRMM patients (median of 2 prior therapies) led to the approval of the drug. A pooled update of both trials at a median follow-up of 48 months confirmed the continuing benefit in overall survival (OS) for the RD arms (RD vs DEX: median OS 38.0 vs 31.6 months; P = .045), although 47.6% of patients in the DEX arm received LEN-based treatment after disease progression or unblinding.17 RD is a category 1 recommendation for RRMM patients per National Comprehensive Cancer Network guidelines.18 LEN-based combination therapies with cytotoxic agents (cyclophosphamide,19 PLD, and vincristine20 ), antibodies (elotuzumab,21 daratumumab22 ), or PIs (BTZ,23 CFZ24 ) resulted in ORRs from 65% to 95% and higher-quality responses taking a step further in improving the outcomes of RRMM patients. The response rates and survival are summarized in Table 2. Chronic LEN-induced diarrhea may be related to bile-acid malabsorption and may respond to reduction in dietary fat intake and/or treatment with bile-acid sequestrants.26,27 The increasing use of LEN (5-10 mg) as maintenance after HDT-SCT implies that many patients will relapse during LEN maintenance. Increasing the dose of LEN to 25 mg with the addition of DEX may induce a response in relatively less aggressive relapse and with low tumor burden. Another strategy that has been examined is the addition of low-dose oral cyclophosphamide to RD in patients progressing during LEN therapy, with reasonable responses.28
Pomalidomide.
POM is a third-generation IMiD agent and the most recently approved antimyeloma drug. Combination of POM and DEX was strongly synergistic in both the LEN-sensitive and LEN-resistant cell lines, inhibiting cell proliferation and inducing apoptosis.29,30 Although the efficacy of POM in LEN-naïve patients has not yet been extensively evaluated, among LEN refractory patients, POM in combination with DEX is able to overcome LEN resistance in ∼30% of patients, with a duration of response between 7 and 8 months. This has been demonstrated in multiple phase 2 studies,31,32 as well as in a large randomized phase 3 trial.33,34 POM can safely combine with CFZ,35 cyclophosphamide,36 and clarithromycin,37 resulting in deeper responses in RRMM patients. Both a subanalysis of the MM003 study and the prospective Intergroupe Francophone du Myélome (IFM) study indicated that POM may be more effective in patients with del(17p) than in patients with t(4;14).38 This is the first drug to show increased activity in del(17p) myeloma and this requires further investigation to incorporate POM in strategies targeting this high-risk population. Additional combination studies on POM are reviewed in Table 3.
PIs.
Bortezomib.
BTZ is a boronate, reversible, first-in-class proteasome inhibitor that primarily targets the constitutive proteasome subunit β5 of the 26S proteasome. BTZ as single agent has resulted in an ORR of 27% at doses of 1.3 mg/m2 given on days 1, 4, 8, and 11 every 21 days.42 Based on the observed synergistic activity with the other agents, various combinations have been evaluated in phase 1/2 trials. BTZ in combination with IMiDS (LEN,23 THAL6 ), alkylating agents (cyclophosphamide,19,43 bendamustine,44 melphalan45 ), anthracyclines (PLD46 ), and histone deacetylase inhibitors (vorinostat47 and panobinostat48 ) resulted in higher ORRs (55%-87%) and can be considered for the management of RRMM. BTZ+DEX (VD) and BTZ+PLD (V-PLD) regimens are National Comprehensive Cancer Network category 1 recommendations for RRMM patients.18 Response rates, PFS, OS, and toxicities of other BTZ combination regimens are summarized in Table 4 to aid in decision making for using BTZ combinations. Retreatment with BTZ is feasible if peripheral neuropathy (PN) is not present and may induce relatively high rates of responses.51,52
Carfilzomib.
CFZ is a second-generation, irreversible epoxyketone analog of YU-101 that inhibits CT-L activity, resulting in sustained proteasomal inhibition. It is the most recently Food and Drug Administration (FDA)-approved PI for the treatment of patients with myeloma who have received at least 2 prior therapies, including the first-generation PI BTZ and an IMiD agent. However, it has not yet been approved by the European Medicines Agency. CFZ combined with DEX resulted in good response rates, even among BTZ-refractory patients.53 CFZ administered in combination with LEN and DEX (CRD) resulted in high-quality responses among RRMM patients.24 Similar superior results with CFZ in combination with POM and DEX (CPD) were seen in a heavily pretreated patient population, with a median of 6 prior lines of therapy.35 A large phase 3 trial, ASPIRE, randomized 792 patients to CRD or RD among relapsed myeloma patients who received a median of 2 prior lines of therapy, demonstrating that the addition of CFZ to LEN and DEX resulted in significantly improved PFS (CRD vs RD: median 26.3 m vs 17.6 m; P = .0001, respectively).54 These impressive results suggest that CFZ may be used much earlier in the relapsed myeloma patients, although the efficacy of BTZ in CFZ-refractory patients at a later time is unclear. Major advantages of CFZ as a choice of PI compared with BTZ are better proteasomal inhibition,55 efficacy in BTZ-refractory patients, and significantly reduced incidence of peripheral neuropathy,56 whereas the disadvantages include consecutive days of administration requiring 6 infusion visits in a 28-day cycle.56 Studies evaluating weekly schedule of CFZ at higher doses are underway (ENDEAVOR study). Another phase 3 study, FOCUS, randomized 315 heavily pretreated RRMM patients to single-agent CFZ or to best supportive care with a steroid and oral cyclophosphamide. The trial did not meet the primary end point and no significant differences in OS were found between the 2 treatment arms. Phase 1 trials of CFZ in combination with ARRY 52057 and panobinostat58 have promising preliminary efficacy, as listed in Table 5. Other CFZ combination studies are also reviewed in Table 5.
Cytotoxic agents.
Before the availability of the newer agents, low-dose oral cyclophosphamide combined with steroids was used as a common regimen for RRMM patients.65 Because of its relative safety in renal failure patients, ease of administration, predictable side effect profile, and the metronomic effect, it was evaluated in combination with BTZ,43 LEN,19 and THAL,9 resulting in good response rates. Combinations of cyclophosphamide have demonstrated activity in RRMM.36 PLD is another cytotoxic agent that can be safely administered in renal failure patients. In combination with BTZ, PLD yielded PR rates ≥45% and gained a 3-month PFS benefit compared with BTZ alone.46 Combination cytotoxic therapies may still have a role in myeloma therapies, especially when the tumor burden is high with an aggressive relapse. Aggressive cytoreduction with a combination chemotherapeutic regimen such as DEX, cyclophosphamide, etoposide, and cisplatin (DCEP)66 with or without BTZ (V-DCEP)67 or a combination such as BTZ, THAL, DEX, cisplatin, doxorubicin, cyclophosphamide, and etoposide (VTD-PACE)68 is a reasonable salvage regimen. During this era where minimizing alkylator therapy is a strong consideration to avoid long-term complications of therapy-related myelodysplastic syndrome or secondary leukemias,69 these effective cytoreductive therapies may have a small role to serve as debulking agents and as a bridge to the next line of therapies including HDT-SCT.
HDT-SCT in the salvage setting.
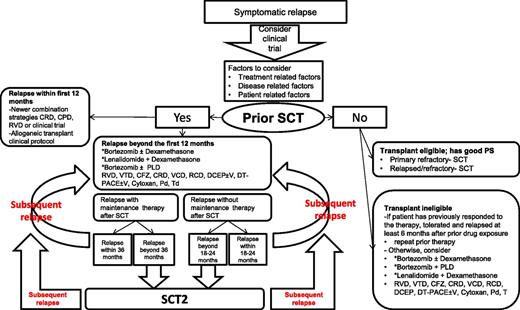
The role of HDT-SCT (HDT-SCT2) in RRMM patients was recently evaluated in a phase 3 study that randomized patients to HDT-SCT2 or cyclophosphamide who progressed ≥18 months after a previous SCT.70 At a median follow-up of 31 months, PFS was 19 months vs 11 months, favoring HDT-SCT. This study provides evidence for the efficacy of salvage HDT-SCT compared with salvage cyclophosphamide (not a common treatment choice), essentially comparing 2 alkylator regimens in RRMM patients. Another large retrospective registry study71 reported risk of progression of 51%, 82%, and 91% at 1, 3, and 5 years, respectively. These analyses were done at the time when the currently FDA-approved newer agents (CFZ, POM) were unavailable and the role of maintenance therapies with LEN and BTZ was not yet established. With the availability of novel agents (BTZ, LEN, CFZ, and POM), the role of a HDT-SCT2 must be weighed carefully against toxicity and activity of the latter novel agents (or their combinations) as salvage therapy. HDT-SCT2 may be a reasonable option to consider for young transplant-eligible patients with good performance status (PS) who derived a long PFS after HDT-SCT1. Among patients not receiving maintenance therapies after HDT-SCT, if the relapse is beyond the expected PFS (18-23 months),71-73 HDT-SCT2 is an option to consider. For patients who have relapsed while receiving maintenance therapy, and the duration of remission is beyond the expected PFS (39-41 months for LEN maintenance72,73 and 35 months for BTZ maintenance),74 HDT-SCT2 may also be offered. The availability of plerixafor may improve results of a second attempt for stem cell collection if cells from the first HDT-SCT are not available. Salvage HDT-SCT can be offered for primary refractory patients as well as RRMM patients if they have good PS and are considered transplant eligible. Figure 1 summarizes the algorithm for HDT-SCT in the salvage setting.
Treatment options for relapsed and refractory myeloma. *NCCN category 1 recommendations; CFZ: carfilzomib; CPD: carfilzomib, pomalidomide and dexamethasone; CRD: carfilzomib, lenalidomide and dexamethasone; DCEP±V: dexamethasone, cyclophosphamide, etoposide, and cisplatin±bortezomib; DT-PACE±V: dexamethasone, thalidomide, cisplatin, doxorubicin, cyclophosphamide, and etoposide±bortezomib; Pd: pomolidomide and dexamethasone; PFS: progression free survival; PLD: liposomal doxorubicin; PS: performance ststus; RCD: lenalidomide, cyclophosphamide and dexamethasone; RVD: lenalidomide, bortezomib and dexamethasone; SCT: autologous stem cell transplant; SCT2: second SCT; Td: thalidomide and dexamethasone; VCD: bortezomib, cyclophosphamide and dexamethasone; VTD: bortezomib, thalidomide and dexamethasone.
Treatment options for relapsed and refractory myeloma. *NCCN category 1 recommendations; CFZ: carfilzomib; CPD: carfilzomib, pomalidomide and dexamethasone; CRD: carfilzomib, lenalidomide and dexamethasone; DCEP±V: dexamethasone, cyclophosphamide, etoposide, and cisplatin±bortezomib; DT-PACE±V: dexamethasone, thalidomide, cisplatin, doxorubicin, cyclophosphamide, and etoposide±bortezomib; Pd: pomolidomide and dexamethasone; PFS: progression free survival; PLD: liposomal doxorubicin; PS: performance ststus; RCD: lenalidomide, cyclophosphamide and dexamethasone; RVD: lenalidomide, bortezomib and dexamethasone; SCT: autologous stem cell transplant; SCT2: second SCT; Td: thalidomide and dexamethasone; VCD: bortezomib, cyclophosphamide and dexamethasone; VTD: bortezomib, thalidomide and dexamethasone.
Allogeneic SCT in the salvage setting.
The role of allogeneic SCT (allo-SCT) as salvage therapy for RRMM remains unclear. Although a small proportion of patients may derive long-term benefit, the mortality and morbidity in the form of acute/chronic graft-versus-host disease associated with allo-SCT strongly outweighs the benefits as viewed by multiple published series to date.75,76 The probability of benefit from an allo-SCT in a patient with aggressive relapse or among patients with high-risk myeloma also remains unproven. Among high-risk patients who achieve a good quality response with salvage therapy, allo-SCT may be considered, although with the high risks of transplant-related mortality, preferably in the setting of a clinical trial and using newer agents like BTZ for graft modulation posttransplant.77,78 Outside the context of well-designed and novel clinical trials, the strategy of allo-SCT for RRMM should not be routinely considered.
Factors influencing treatment choices at relapse
Several factors influence the choice of therapy at relapse (Figure 1). The choice of a salvage regimen should offer a good balance between the efficacy and toxicity of the regimen. Disease-related factors such as the risk stratification of the disease, any acquired chromosomal aberrations; treatment-related factors such as prior drug therapy, regimen-related toxicity such as peripheral neuropathy, and myelosuppression; and the depth and duration of response to prior drugs should be taken into consideration. Patient-related factors such as renal and hepatic impairment, comorbidities, susceptibility to infections, and PS of the patient should all be reflected on the choice of salvage therapy.
Optimal timing to initiate therapy at relapse
When patients present with biochemical relapse in the absence of hypercalcemia, renal failure, anemia, or bone lesions (or CRAB) criteria, the optimal timing of initiating salvage treatment is always questionable. Previous complications of the disease (ie, presentation with myeloma-related renal impairment, extramedullary disease) may indicate an earlier rather than a later time of initiation of therapy, before symptom development. In the absence of evidence of high tumor burden or an aggressive relapse (with elevated serum lactate dehydrogenase,79 rapidly rising paraprotein levels,2 light-chain escape80 ), observation of biochemical relapse with monitoring every 6 to 8 weeks until the patient develops clinical manifestations of symptomatic myeloma is currently recommended.
Disease-related factors
Impact of cytogenetic abnormalities.
Decision making on salvage therapy in genetically high-risk RRMM patients revealing chromosomal abnormalities t(4;14) and del(17p) is difficult because of the absence of prospective studies. The prognostic importance of these high-risk cytogenetics in the up-front setting may be different compared with their prognostic importance at relapse. Thus, studies in newly diagnosed patients suggest that newer agents or combinations of newer agents may partly overcome the adverse prognosis conferred by the poor-risk genetics. However, the data are still conflicting. The use of BTZ in the Total Therapy 3 program improved the prognosis of patients with t(4;14) or del(17p). In a study from the IFM, with 106 and 54 patients exhibiting t(4;14) and del(17p), respectively, patients treated with BTZ with t(4;14) or del(17p) displayed a shorter event-free survival and overall survival (OS) than did those not harboring these cytogenetics. When compared with high-risk patients treated with a vincristine, adriamycin, dexamethasone induction, it was shown that BTZ improved both the event-free survival and OS of patients with t(4;14) but not of patients with del(17p).81 Data from the HOVON-65 study indicated that the presence of t(4;14) was associated with worse PFS and OS, although patients in the PAD (BTZ, adriamycin, and DEX) arm achieved better results in this group of patients, though statistical significance was not reached. In patients with del(17p13), both PFS and OS were significantly better in the PAD arm.74 Several retrospective studies evaluated the impact of cytogenetic abnormalities among patients with RRMM. In a Canadian study, treatment with LEN/DEX resulted in a PFS of 2.2 months and an OS of 4.7 months in patients with del(17p) and time to progression (TTP) of 8 months with t(4;14).82 LEN/DEX neither overcame the negative impact of t(4; 14) from the French (IFM) analysis83 nor the negative impact of del(17p) from a Greek prospective study.79 Thus the data on the ability of LEN/DEX to overcome poor risk genetics is mixed among retrospective trials. However, the third-generation IMiD drug POM presents different results. In the MM-003 study, which compared POM in combination with low-dose DEX (LoDEX) vs high-dose DEX (HiDEX), POM+LoDEX was superior to high HiDEX in terms of ORR, PFS, and OS in patients with poor-risk cytogenetics. Median OS for high-risk cytogenetics vs standard risk on the POM+LoDEX was 9.9 vs 14.1 months, respectively.84 In a prospective study from the IFM, which included RRMM patients with del(17p) or t(4;14), POM therapy was associated with longer PFS in patients with del(17p) than in patients with t(4;14), suggesting improved efficacy of POM+LoDEX among patients with del(17p).38 Deeper responses were observed in a heavily pretreated RRMM patient population in another combination study of POM with BTZ and DEX, which included high-risk patients, suggesting that combinations of POM and PI probably represent the current best approach for high-risk myeloma in the relapsed setting, though data are limited at this time.40 To summarize, current data on newer agents indicate that they may only partly overcome the deleterious impact of high-risk cytogenetics in the relapsed setting. There is an urgent need for the development of novel and innovative strategies for the treatment of patients with high-risk cytogenetics. Secondary translocations and mutations of RAS or FGFR3, MYC dysregulation, deletion in p18, or loss of expression or mutation in TP53 may play a role in determining tumor progression and drug resistance.85 Emerging data suggest that there may be a role in determining these abnormalities at relapse, especially the presence of del(17p). Patients with such high-risk features should be strongly encouraged to participate in clinical trials of new agents.
Treatment-related factors
Prior drug exposures and regimen-related toxicity.
Reviewing the entire treatment history and tolerability to prior drug exposures is very crucial before choosing a salvage regimen for RRMM patients. Patients who have progressed on the IMiD, PI, cytotoxic doublet or triplet combination therapies (RD, VD, TD, BTZ+TD [VTD], cyclophosphamide+TD [CTD], BTZ+RD [RVD], cyclophosphamide+BTZ+D [VCD], cyclophosphamide+RD [RCD], V-PLD) therapy can be offered treatment with next-generation regimens such as (CFZ+DEX [CD], POM+DEX [PD], CRD, CPD, cyclophosphamide+CD [CCD], POM+CD [PCD], or POM+VD [PVD]). Patients that are PI- or IMiD-naïve should be offered a regimen they have not been previously exposed to, based on the side effect profile and taking into account the nature of prior therapies. Avoiding the agents that have resulted in prior regimen-related toxicity and choosing a tolerable salvage therapy are essential. For example, V-PLD among RRMM patients with heart failure may not be the best choice of salvage therapy because of the potential for a decrease in left ventricular ejection fraction in 13% of patients in the phase 3 study comparing V-PLD with BTZ.46 Similarly, avoiding BTZ among patients with existing ≥G3 PN or ≥G2 with pain and instead choosing a CFZ-based regimen will mitigate the potential toxicity. The principle of combining agents with nonoverlapping toxicities such as PN or myelosuppression will enable the selection of a tolerable effective salvage regimen. Once the regimen is chosen, close monitoring with appropriate dose reductions, as summarized in Table 6, will result in uninterrupted delivery of the myeloma agents aimed at disease control.
Longevity of prior remission.
The longevity of the prior remission duration is a critical component in making a choice of salvage therapy. The depth of the first response, remission duration of the patient’s prior therapies, and tumor burden at relapse can suggest the aggressiveness of the relapse. A relapse within the first 12 months after an early transplant should be treated very differently by using an intense combination such as a PI plus a novel IMiD such as RVD,23 or novel approaches with CRD54 or CPD,35 or similar (based on the availability of the agents), compared with patients who relapse later in the course.91 These patients should be strongly encouraged to participate in clinical trials evaluating new agents with novel mechanisms of actions and may be considered for allo-SCT on a clinical protocol.
Retreatment with prior therapies.
Data from the VISTA92 and MM-01593 studies indicate that reuse of BTZ or LEN, respectively, is associated with a response rate of ∼50% to 60%, especially in patients who initially responded to VMP or MPR, respectively. The RETRIEVE trial, a phase 2 trial, evaluated the role of retreatment with BTZ among patients who had previously responded to BTZ treatment and relapsed at least 6 months after prior BTZ exposure. Among 130 RRMM patients who received a median of 2 prior therapies (coadministration with DEX in 72% patients), the ORR was 40% and TTP among the responding patients was 8.4 months.52 A general recommendation is to consider retreatment with a prior agent if the patient has previously responded to the treatment and relapsed at least 6 months after prior drug exposure and tolerance was acceptable.
Patient-related factors
Renal insufficiency.
RRMM patients may present with renal insufficiency (RI) either caused by disease progression or by other predisposing conditions (eg, diabetes, hypertension, vascular disease, use of nephrotoxic drugs) that are common, especially in patients of advanced age. The presence of renal dysfunction may have an impact on the treatment decisions. Both the PIs BTZ94 and CFZ56 can be used without dose adjustments. Among IMiDs, THAL95 does not require dose adjustments; LEN requires dose adjustments, and for POM there are preliminary data that indicate safety in patients with RI even at standard doses.96,97 POM is currently being prospectively evaluated in patients with severe RI in the MM-008 (US) and MM-013 (EU) trials. Among the cytotoxic treatments commonly used currently, cyclophosphamide and PLD do not require dose adjustments, whereas melphalan does. Table 6 suggests recommended renal dose adjustments for individual agents for regimen-related toxicities. When considering combining therapies, the same guiding principles should be used to determine the choice of therapy. The combination of a PI and steroids with cyclophosphamide43 or THAL/renal dose–adjusted LEN is recommended based on the patient’s tolerability.98,99
Hepatic impairment.
It is not uncommon for RRMM patients to have hepatic impairment (HI) as a result of concomitant medications taken for pain control or other comorbidities or from regimen-related toxicity. This severely influences the treatment choices. Limited data exist for patients with a bilirubin level >2 upper limit of normal, or patients with Child-Pugh stage 2 or 3. Table 6 suggests dose reductions for the newer agents and combination therapies.
Comorbidities.
The advanced age at diagnosis and the concurrent comorbid conditions in elderly patients such as heart disease and diabetes complicates the decision making in RRMM management. The age-related decline in physiologic reserve and the decline in organ function may potentially make them more vulnerable for toxicity. Assessing vulnerability as defined by the European Myeloma Network, anticipating regimen-related toxicities and individualizing therapy based on their risk factors (GO-GO, MODERATE-GO, SLOW-GO) would allow for effective delivery of therapy without interruptions and also allow for long-term management.85,100
Emerging therapies
Newer proteasome inhibitors
Ixazomib.
Ixazomib (MLN9708) is a reversible oral boronate peptide, a next-generation PI that is farthest in clinical development and closer to approval. Ixazomib is pharmacokinetically and pharmacodynamically distinct from BTZ, with superior tissue penetration and greater biological activity.101 Weekly and twice-weekly schedules of ixazomib have been evaluated among RRMM patients, and preliminary data suggest clinical activity of single-agent ixazomib. From two phase 1 trials with ixazomib as the single agent, the maximum tolerated dose (MTD), schedule of administration, and safety profile have been established. One trial administered MLN9708 on a twice-weekly basis, similar to BTZ administration (days 1, 4, 8, 11), of a 21-day cycle.102 The second trial administered MLN9708 once weekly for 3 of 4 weeks (days 1, 8, 15) of a 28-day cycle. The drug is well tolerated, with a remarkably lower toxicity profile, with low rates of PN observed.103 In combination with LEN and DEX, twice-weekly dosing of ixazomib was well tolerated and exhibited deeper responses. These results are encouraging for the possibility of a highly efficacious, oral triplet regimen in the induction therapy for those newly diagnosed with MM.104
Marizomib.
Marizomib (NPI0052) is an irreversible, nonpeptide, orally available PI that inhibits CT-L and T-L activities at much lower concentrations, although higher concentrations are required to inhibit C-L activity.105 A phase 1 trial of marizomib administered twice weekly (days 1,4,8, and 11 over 21 days) in 34 RRMM and predominantly BTZ-refractory (71%) patients yielded PR rates ≥20%.106 The MTD for infusion over an hour is 0.4 mg/m2 and for infusion over 2 hours is 0.5 mg/m2.107 Combination trials are currently under evaluation.
Oprozomib.
Oprozomib (ONX0912) is an oral, abbreviated derivative of the irreversible PI CFZ. Similar to BTZ, it predominantly inhibits CT-L activity of the β5 subunit of the proteasome. Among the 30 RRMM patients and 12 Waldenström macroglobulinemia patients treated on a twice-weekly (2/7) schedule or 5 days every 2 weeks (5/14). Two cases of dose-limiting toxicity (DLT), both occurring on the 5/14 schedule were seen (renal failure and tumor lysis syndrome). Currently, dose escalation is continuing in the 2 days out of 7 (300mg/day) and 5 days out of 14 (270 mg/day) dosing schedules. Preliminary data suggest that oprozomib monotherapy has promising activity.108
HDAC inhibitors
Histone deacetylase inhibitors (HDACi) prevent deacetylation, which is a process involved in the epigenetic regulation of gene expression–promoting cell proliferation and cell death. In myeloma cells, HDACi inhibit cell growth and induce apoptosis as a single agent and are also synergistic with BTZ.109 Clinically, the activity of HDACi as single agents is limited, but when combined with DEX or BTZ in RRMM patients, HDACi are able to overcome BTZ resistance. This may possibly be the result of simultaneous targeting of intracellular proteolytic pathways—the ubiquitin-proteasome pathway by BTZ and the aggresome protein degradation pathway by HDACi. Several class-specific inhibitors or pan-deacetylase inhibitors110 are under evaluation.
Vorinostat.
The combination of vorinostat with BTZ (VANTAGE-095) among heavily pretreated RRMM patients resulted in partial response (PR) rates in ≥17% patients and clinical benefit rate in 31% patients. Median OS was 11.23 months and the 2 year OS rate was 32%.111 The VANTAGE-088 randomized 637 patients to vorinostat and BTZ or BTZ alone among early relapsed patients. Median PFS was 7.63 vs 6.83 months (P = .01; hazard ratio 0.77), favoring the vorinostat group. Adverse effects included ≥G3 thrombocytopenia rates of 45% vs 24%.50 Although the combination of vorinostat and BTZ prolonged PFS and reduced the risk of progression by 23% relative to BTZ, the clinical relevance of the difference in PFS between the 2 groups is small per investigators.50 Although this combination offers a new therapeutic option for a difficult-to-treat patient population, their overlapping toxicities are concerning. Alternating the schedule of BTZ or a combination with CFZ may be evaluated to mitigate toxicity while retaining the clinical benefit.
Panobinostat.
Phase 1 studies evaluating panobinostat and BTZ in RRMM patients identified panobinostat 20 mg given on days 1 to 14 and BTZ 1.3 mg/m2 given on days 1, 4, 8, and 11 every 21 days as the MTD.112 In the expansion phase, the majority of patients responded to treatment (ORR 73.3%); however, ≥G3 thrombocytopenia was higher at 89%. The dose-expansion phase, with a week off for panobinostat, was associated with a lower incidence of ≥G3 thrombocytopenia and improved platelet recovery. In the subsequent phase 2 trial, PANORAMA-2, evaluating the combination of panobinostat with BTZ and DEX (VD), ORR of 34.5% and median PFS of 5.4 months were observed, and ≥G3 thrombocytopenia and diarrhea rates were 60% and 20%, respectively (Table 4). This combination can recapture the responses of BTZ-refractory RRMM patients.48 This trial was followed by a large randomized phase 3 trial, PANORAMA-1, that randomized 768 patients to receive panobinostat and VD, or VD alone, in an early-relapsed-disease setting. Panobinostat 20 mg by mouth or matching placebo 3 times per week on days 1 to 14 was given in combination with twice-weekly VD in treatment phase 1 (TP1) for cycles 1 through 8. In TP2 (cycles 9-12), patients received weekly VD. The median PFS was 12 vs 8.1 months (P < .0001), with a hazard ratio of 0.63, favoring the use of panobinostat.113 Among patients able to complete TP2 in the panobinostat arm, the response rates were higher; had longer median PFS114 ; and rates of grade 3/4 adverse events in TP1 vs TP2 were diarrhea (25.5% vs 8.8%), thrombocytopenia (47.1% vs 5.9%), and fatigue (14.7% vs 2.0%), supporting once-weekly administration of BTZ when used in combination with panobinostat and dexamethasone.114,115
ACY-1215.
Rocilinostat (ACY1215) is a selective inhibitor of HDAC-6, developed to minimize the toxicities associated with the other pan-DACi, yet maintaining the efficacy. Similar to other agents in this class, the efficacy of rocilinostat as monotherapy is modest, and the results of a phase 1/2 trial investigating rocilinostat in combination with BTZ and low-dose DEX in heavily pretreated patients demonstrated promising synergy among BTZ-refractory patients.116 Another similar phase 1/2 trial of rocilinostat in combination with LEN and low-dose DEX in heavily pretreated patients is underway.117
KSP inhibitors
ARRY-520.
ARRY-520 is a kinesin-spindle protein (KSP) inhibitor that induces cell death by targeting the KSP and inhibiting spindle formation during mitosis. As a single agent, ARRY-520 has modest activity, with an ORR of 16%, but in combination with low-dose DEX, antimyeloma agents, it is active in very-heavily-pretreated patients who are triple refractory to BTZ, LEN, and DEX (ORR 28%).118 ARRY-520 is active in combination with BTZ, and DEX above the doses of ≥1.25 mg/m2 per day ARRY-520, with a standard 1.3 mg/m2 per day dose of BTZ, where 31% PRs were seen.119 Hematologic toxicity of 15% ≥G3 neutropenia in this heavily-pretreated patient population can be managed with prophylactic granulocyte colony-stimulating factor support. More importantly, survival for patients with low acute-phase protein a-1 acid glycoprotein (AAG) levels was 23 months compared with 4.5 months among higher baseline AAG levels, suggesting that candidate and agent selection to deliver maximum benefit for a particular treatment is a possibility in the future.120
Monoclonal antibodies
Elotuzumab.
Elotuzumab is a humanized monoclonal antibody that targets the cell surface glycoprotein CS1 (SLAMF7). Elotuzumab mediates ADCC in myeloma cell lines and myeloma cells, from patients with MM that is resistant or refractory to conventional therapies and BTZ.121 Elotuzumab as a single agent demonstrated no objective responses, but in a phase 1 study, in combination with LEN 25 mg, and DEX 40 mg, objective responses were observed in 23 of 28 treated patients (82%).122 DLTs were not found up to a dose of 20 mg/kg, leading to a phase 2 extension phase, where the response rates for elotuzumab doses of 10 mg/kg vs 20 mg/kg were 92% vs 76% and median PFS was 33 vs 26 months, respectively.21 Based on these encouraging results, 2 ongoing phase 3 trials (ELOQUENT 1 and ELOQUENT 2) are comparing the efficacy and the safety of LEN plus low-dose DEX with or without 10-mg/kg elotuzumab in patients with newly diagnosed myeloma and RRMM patient, respectively, for the end points of PFS, ORR, and OS. Elotuzumab combined well with BTZ in a phase 1 dose-escalation study, and no DLTs were observed during the first treatment cycle in patients who received 2.5 to 20 mg/kg elotuzumab. ≥ PR was seen in 48% of patients and responses were seen in BTZ-refractory patients.123 This impressive monoclonal antibody with encouraging antimyeloma activity in RRMM patients is the closest to approval.
Daratumumab.
Daratumumab is a humanized anti-CD38 antibody that not only directly targets tumor cells but also effectively mediates the killing of CD38-expressing plasma cells via antibody-dependent cell-mediated cytotoxicity (ADCC), antibody-dependent cellular phagocytosis, complement-dependent cytotoxicity, and apoptosis. As a single agent, daratumumab has confirmed antitumor activity. In a phase 1 dose-escalation study, heavily pretreated RRMM patients were treated with single-agent daratumumab with doses ranging from 0.005 mg/kg to 24.0 mg/kg. A marked reduction in paraprotein and bone marrow plasma cells was observed at doses ≥4 mg/kg. Overall, 42% of this heavily pretreated population of patients achieved ≥ PR at doses ≥4 mg/kg.22,124 The combination of LEN and daratumumab demonstrated enhanced natural killer–mediated cytotoxicity in vitro using ADCC assays.125 Based on this rationale, a phase 1/2 study of daratumumab in combination with LEN and oral DEX in the RRMM patients was initiated, with the primary objective of establishing the safety profile. Daratumumab dosing ranged from 2 mg/kg to 16 mg/kg weekly for 8 weeks, twice per month for 16 weeks, and once per month until disease progression. Among the reports of 20 patients, preliminary safety data show a manageable safety profile in line with what has previously been reported for LEN. An ORR of 75% with this combination is encouraging.126 Daratumumab was given the breakthrough status, and multiple trials are ongoing for a rapid approval.
SAR650984.
SAR650984 is another humanized anti-CD38 antibody with strong proapoptotic activity independent of cross-linking agents. Killing is mediated by ADCC, antibody-dependent cellular phagocytosis, and complement-dependent cytotoxicity.127 In a phase 1 dose-escalation trial SAR is administered as a single agent IV infusion every week or every 2 weeks to adult patients with selected CD38+ hematologic malignancies, with doses ranging from 0.0001 mg/kg every 2 weeks to 10 mg/kg every week. MTD was been reached and DLTs were limited to G2 infusion reactions. Among the 6 MM patients treated at the dose of 10 mg/kg administered every 2 weeks, 3 patients had PR and 2 had stable disease.128 With a manageable safety profile, further combination strategies can be planned.
Summary
Given the many different treatment options in clinical practice, as well as the rapid development of many new targets and agents, how to synthesize the large amount of clinical data at any given point remains a clinical challenge. Figure 1 represents a generalized overview of considerations, including when to consider a patient for a transplant, and perhaps a way forward for patients who are not enrolling in a clinical trial. As with all suggested guidelines, this is not meant to be encompassing, but rather an option for approaching patients with RRMM. This represents loose guidelines for treatment approaches and obviously will vary based on access to drugs and the specific individual situation a patient faces at each step of the process.
Conclusions
The long-awaited durable remissions are now within easier reach with successful integration of the available agents. The enhanced antimyeloma activity of some monoclonal antibodies as single agents is encouraging. The lesser toxicity makes them amenable for successful integration with other agents and it is hoped will augment the response rates. Outside of known clinically proven targets, taking advantage of the cell-signaling pathways, the bone marrow microenvironment factors (eg, hypoxia, angiogenesis, the malignant plasma cell-bone marrow stroma interactions) may be potential targets for future drug development. In the course of finding newer targets, a much better understanding of the complex issue of drug resistance mechanisms in myeloma is necessary. Using biomarkers to guide the optimal choice of salvage therapy would open doors for personalizing treatment options for the myeloma patients that would entail lesser toxicities and improve the responses that ultimately are aimed at improving the long-term remissions and survival of myeloma patients.
Acknowledgments
S.L. is supported in part by the Levine Family Fund for Multiple Myeloma Research.
Authorship
Contribution: A.K.N., E.K., M.A.D., and S.L. were all involved in the review of the literature, writing, and organizing of the manuscript.
Conflict-of-interest disclosure: A.K.N. sits on the advisory board for Onyx. M.A.D. is a consultant for Celgene, Onyx, and Ortho-Biotech. S.L. is a consultant for Millennium, Celgene, Novartis, BMS, Onyx, Sanofi, and Janssen. The remaining author declares no competing financial interests.
Correspondence: Sagar Lonial, Department of Hematology and Medical Oncology, Winship Cancer Institute, Emory University, 1365 Clifton Rd, Building C, Room 4004, Atlanta, GA 30322; e-mail: sloni01@emory.edu.