Key Points
Absence of NETs in PAD4−/− mice did not affect bacteremia in polymicrobial sepsis produced by cecal ligation and puncture.
PAD4-deficiency improved outcome in lipopolysaccharide (LPS)-induced sepsis.
Abstract
Neutrophil extracellular traps (NETs), consisting of nuclear DNA with histones and microbicidal proteins, are expelled from activated neutrophils during sepsis. NETs were shown to trap microbes, but they also fuel cardiovascular, thrombotic, and autoimmune disease. The role of NETs in sepsis, particularly the balance between their antimicrobial and cytotoxic actions, remains unclear. Neutrophils from peptidylarginine deiminase 4-(PAD4−/−) deficient mice, which lack the enzyme allowing for chromatin decondensation and NET formation, were evaluated. We found that neutrophil functions involved in bacterial killing, other than NETosis, remained intact. Therefore, we hypothesized that prevention of NET formation might not have devastating consequences in sepsis. To test this, we subjected the PAD4−/− mice to mild and severe polymicrobial sepsis produced by cecal ligation and puncture. Surprisingly, under septic conditions, PAD4−/− mice did not fare worse than wild-type mice and had comparable survival. In the presence of antibiotics, PAD4-deficiency resulted in slightly accelerated mortality but bacteremia was unaffected. PAD4−/− mice were partially protected from lipopolysaccharide-induced shock, suggesting that PAD4/NETs may contribute to the toxic inflammatory and procoagulant host response to endotoxin. We propose that preventing NET formation by PAD4 inhibition in inflammatory or thrombotic diseases is not likely to increase host vulnerability to bacterial infections.
Introduction
Sepsis remains a significant health care problem with approximately 750 000 cases per year, leading to death in 30% of patients in the United States.1 The pathologies of sepsis result not only from the presence of an infection, but also from the hyperinflammatory host response.2 The vast systemic effects seen in sepsis result in diagnostic criteria that are broad in nature.3 Severe sepsis, characterized by organ dysfunction and septic shock, accompanied by hypotension, can rapidly progress to an irreversible stage in which survival is not possible despite therapeutic intervention.2,3
Neutrophil extracellular traps (NETs) are the result of a coordinated biological process whereby neutrophils release their nuclear DNA accompanied by many antimicrobial proteins, including histones.4-6 The first report identified NETs in an infected appendix.4 Using animal models of sepsis, the release of NETs within the vasculature became evident.6-8 Their deposition in organs and prothrombotic activity may contribute to organ failure.9-11 NET biomarkers are elevated in septic patients.12-14 Microbes trapped within NETs are sometimes killed,4,15 and thus NETs could represent an important mechanism of host defense, particularly in sepsis.7,16-18 To date, this has not been rigorously tested.
NETs have been identified in the cecal ligation puncture (CLP) mouse model of polymicrobial sepsis.19,20 Deoxyribonuclease 1 (DNase 1) degrades NETs,4 and one study suggests that DNase infusion results in increased susceptibility to death in CLP.19 However, this effect was transient and minor, with higher mortality at 24 hours but similar mortality at subsequent time points. Although bacterial loads were elevated in DNase-treated mice 6 hours after CLP, by 24 hours colony forming units (CFUs) were similar between treated and untreated mice.19 Histologic evidence of increased organ damage was evident by 24 hours19 and could be due to liberation of NET fragments by DNase 1 having a cytotoxic effect on distant tissues. On the other hand, DNase 1 naturally facilitates clearance of NETs by macrophages,21 diminishing toxic NET effects. A recent study showed the opposite result with a similar method of DNase administration.22 It is important to note that CLP experiments are difficult to compare because the degree of sepsis is dependent on the amount of spillage of cecal contents into the peritoneum, and because animals housed in different facilities at different institutions likely have differences in gut microbiota. For this reason, in our present study, we used siblings from the same litters.
The release of NETs within the bloodstream has important procoagulant and prothrombotic implications.16,23 NETs can bind platelets and red blood cells,23 and thus participate in the initiation of pathological thrombosis.24,25 Peptidylarginine deiminase 4 (PAD4) is important for chromatin decondensation during NETosis by modifying histone charges through citrullination.26,27 We have seen significant antithrombotic and cardioprotective effects in the absence of NETs28,29 using PAD4−/− mice, which do not decondense chromatin or form NETs.26 Notably, in our colony, these mice do not suffer from opportunistic infections.
The most abundant proteinaceous components of NETs are histones,30 which are themselves not only procoagulant9,31-33 but also highly cytotoxic to endothelium.9,34,35 The hypercoagulable state and organ dysfunction exacerbated by histones, some of which may originate from NETs, can quickly lead to host mortality.9 Therefore, it is important to study sepsis in PAD4−/− mice, which have a defect in NET formation, in order to further delineate the balance between antimicrobial host defense and the pathological consequences of NET release. In this study, we report that mice lacking PAD4 fared the same or better than wild-type (WT) mice in mouse models of sepsis, except in the presence of antibiotics where there was a slight acceleration in mortality. Unexpectedly, we found that there was no difference in bacteremia in polymicrobial sepsis between PAD4−/− and PAD4+/+ mice under any condition.
Methods
Study approvals and animals
All experimental procedures involving animals were reviewed and approved by the Institutional Animal Care and Use Committee of Boston Children’s Hospital (protocol numbers: 11-04-1848 and 14-03-2631R). All mice received food and water ad libitum; for in vivo sepsis models, mice were provided Napa Nectar enrichment gel (SE Laboratory Group). Mice from our PAD4−/− colony, backcrossed for 9 to 11 generations to C57BL/6J background, were continually backcrossed at least once per year to C57BL/6J mice ordered from the Jackson Laboratory. Littermates from heterozygous crosses were used in all CLP experiments.
Flow cytometry
Reactive oxygen species (ROS) formation and degranulation analyses were performed using flow cytometry (BD FACSCantoII) and analyzed using FloJo software version 8.8.7 (Tree Star Inc., Ashland, OR). Whole blood was collected using heparin-coated capillary tubes via the retro-orbital sinus and stimulated as previously described in the presence of dihydrorhodamine-123 (Invitrogen).36 After stimulation, red blood cell lysis, and several washing steps, cells were stained using Alexa Fluor 647-conjugated anti-Ly6G antibody (BioLegend) for ROS measurements, and Alexa 647-conjugated anti-Ly6G antibody (BioLegend) and fluorescein isothiocyanate-conjugated LAMP-1 antibody (BD Pharmingen) for degranulation measurements. Neutrophils were gated by forward-and-side scatter and followed by Ly6G gating. Rhodamine+ neutrophils were quantified for ROS production and Lamp1+ neutrophils were quantified for degranulation studies.
Thioglycollate peritonitis
Thioglycollate broth (3% in phosphate-buffered saline; Sigma-Aldrich) was prepared at least 2 weeks prior to use. One mL of the broth was injected IP per mouse. At the indicated time points, mice were euthanized and a peritoneal lavage was performed using 6 mL of phosphate-buffered saline. Total cell counts were performed using a Neubauer hemocytometer and a portion of the infiltrate was cytospun for differential cell counting using a StatSpin CytoFuge 2 (Beckman Coulter). Differential counts were performed using at least 200 cells stained with the Wright-Giemsa histologic stain.
CLP
CLP surgery was performed as described37 using aseptic surgical technique. All mice in CLP experiments were obtained by heterozygous crosses, and therefore were littermates on a C57BL/6J background. Mice were anesthetized using 3.5% isoflurane in 100% oxygen and maintained at 2% isoflurane for the duration of the surgery. A midline laparotomy allowed for access to the cecum. The cecum was ligated with 4-0 silk suture located at either 50% (low-grade) or 75% (high-grade) between the iliocecal junction and the distal end of the cecum. A through-and-through puncture was performed using a 21G (low-grade) or 18G (high-grade) needle. A small amount of cecal contents were extruded to ensure hole patency before returning the cecum to the abdominal cavity. The peritoneum was closed with 6-0 monofilament absorbable vicryl suture and the skin was closed using a 6-0 nylon suture. Immediately after surgery, mice were injected with 0.9% sterile saline (500 μl SC) for fluid resuscitation. Mice received buprenorphine (0.05 mg/kg SC) as an analgesic immediately prior to surgery and every 8 to 12 hours subsequently, for 72 hours. Sham animals underwent the same procedure except for the ligation and puncture of the cecum. Control animals did not undergo surgery and were euthanized alongside CLP animals. All surgeries were done at similar times of the day to account for possible circadian rhythm effects on the model. Mice were observed over a 96-hour (low-grade CLP) or 10-day (high-grade CLP) time period and assessed for moribundity, along with body weight and temperature. For mice receiving antibiotics, high-grade CLP was induced and antibiotics (imipenem/cilastatin, 25 mg/kg injected IP) were given starting 4 to 6 hours after surgery and continuing every 12 hours until the end of the study. Temperatures were recorded using a handheld, noncontact infrared thermometer (Kintrex). All mice exhibited clinical sepsis symptoms (lethargy, piloerection, reduced interest in food and water, and hunched posture). Any mice exhibiting loss of righting reflex when placed in a supine position were identified as moribund and immediately euthanized.
Lipopolysaccharide (LPS) endotoxemic shock
LPS from Salmonella enterica spp typhimurium (Sigma-Aldrich; Lot #109K4087) was injected IV via the retro-orbital sinus at doses of 25, 10, or 2.5 mg/kg. Lethal dosing was determined by injecting mice with increasing amounts of LPS and observing mortality over a 24-hour period. Mice were observed over a 96-hour time period and assessed for morbidity, along with body weight and temperature. Mice received buprenorphine (0.05 mg/kg SC) as an analgesic every 12 hours. Temperatures were recorded using a handheld, noncontact infrared thermometer (Kintrex). All mice exhibited clinical symptoms of endotoxemic shock (lethargy, piloerection, reduced interest in food and water, and hunched posture). Any mice exhibiting loss of righting reflex when placed in a supine position were identified as moribund and immediately euthanized.
Complete blood counts
Blood was collected via the retro-orbital sinus into EDTA-coated capillary tubes. Blood cell counts were analyzed using a Hemavet 950FS Veterinary Multi-species Hematology System (Drew Scientific).
Plasma measurements
Blood was collected into sodium citrate anticoagulant (10% v/v). After centrifugation of whole blood at 6000 rpm for 5 minutes, plasma was collected and centrifuged at 13 200 rpm for 5 minutes to remove remaining cellular components. DNA was measured using the PicoGreen Quant-iT dsDNA Assay Kit (Invitrogen). Thrombin anti-thrombin (TAT) complexes (Abcam), soluble P-selectin (sPsel) (R&D Systems), interleukin (IL)-6 (R&D Systems), IL-10 (R&D Systems), and alanine aminotransferase (Biotron) were measured according to the manufacturer’s instructions.
Bacterial load
All blood, lavage, and tissue collection and homogenization were performed using aseptic technique. Serial dilutions were plated onto tryptic soy agar prepoured petri dishes supplemented with 5% sheep’s blood (Hardy Diagnostics), and incubated aerobically overnight at 37°C. Plates with 20 to 200 colonies were counted and CFUs were normalized per gram of tissue collected.
Circulating histone analysis
Four microliters of plasma were denatured and resolved on Tris-glycine SDS-Page gels (Lonza) or Bis-tris glycine gels (Invitrogen) under reducing conditions. After semi-dry transfer to polyvinylidene difluoride membrane, blots were blocked with 3% bovine serum albumin for 3 hours at room temperature (RT). After being incubated in primary antibody overnight at 4°C (anti-citrullinated histone H3 [H3Cit], Abcam, 1:1500) or for 1 hour at RT (anti-histone H3, Abcam, 1:6000), membranes were washed and incubated in horseradish peroxidase-conjugated secondary antibody (goat anti-rabbit IgG horseradish peroxidase-conjugate, Bio-Rad, 1:20000) for 1 hour at RT. After washing, Pierce enhanced chemiluminescence substrate (Thermo Scientific) was applied to each membrane before exposure onto film. H3Cit blots were exposed for 10 minutes, while histone H3 blots were exposed for 1 minute.
Statistics
Data are presented as mean ± SEM and analyzed using Student t test or Mann-Whitney U test unless otherwise noted. Survival data were analyzed using Log-Rank tests of Kaplan-Meier curves. Data were considered significant when P values were < .05.
Results
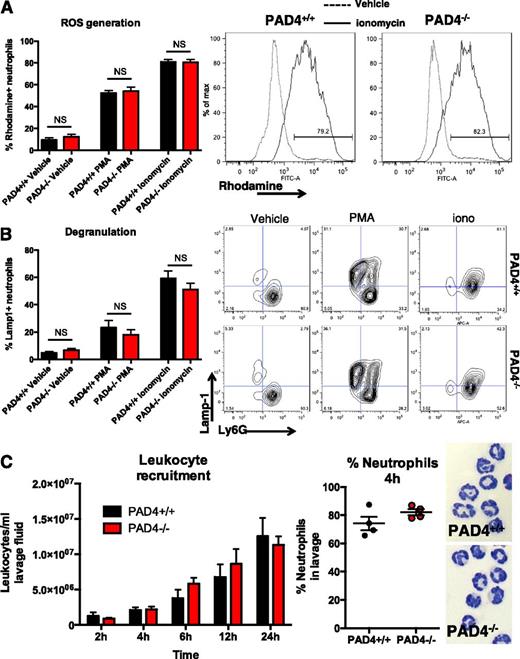
PAD4−/− mice have circulating neutrophil numbers similar to WT PAD4+/+ mice and their bacterial killing ability by phagocytosis is not impaired.26 PAD4−/− mice are highly impaired in their ability to generate NETs, even in response to ROS-dependent stimuli such as phorbol myristate acetate (PMA).26 Exogenous H2O2 fails to generate NETs in vitro in PAD4−/− neutrophils,26 as does calcium increase by ionophores.28 This highlights the importance of histone citrullination-mediated chromatin decondensation during NETosis. We found that PAD4−/− neutrophils are capable of producing ROS, as H2O2 generation occurs in PAD4−/− neutrophils (Figure 1A) and show that the ability to degranulate is not affected by PAD4-deficiency (Figure 1B). Leukocyte rolling along activated endothelium occurs normally in PAD4−/− mice.28 To investigate the ability of neutrophils to extravasate in response to an inflammatory stimulus, we quantified leukocyte recruitment to the peritoneum in a model of thioglycollate-induced peritonitis. PAD4−/− mice recruited similar numbers of leukocytes as PAD4+/+ mice over time, and neutrophil recruitment was also not impaired as determined by quantification of cells with characteristic neutrophil nuclear morphology (Figure 1C). Therefore, the use of PAD4−/− animals for sepsis studies appears to provide an ideal model in which to examine the role of NETs, because neutrophils can be recruited and are otherwise competent in non-NET microbicidal functions.
Neutrophils from PAD4−/− mice are competent in non-NET functions. (A) Diluted, anticoagulated whole blood was incubated with 1 μM ionomycin, 100 nM PMA, or vehicle for 20 minutes at 37°C in the presence of dihydrorhodamine-123. Rhodamine+ ROS-generating neutrophils are quantified for each condition (left panel) and representative plots are shown (right two panels); n = 4-13. (B) Diluted whole blood was preincubated with 5 mM cytochalasin B for 20 minutes, then incubated with 1 μM ionomycin, 100 nM PMA, or vehicle for 10 minutes at 37°C. Lamp1+ Ly6G+ degranulated cells are quantified in the left panel, with representative plots shown in the right panels; n = 4-13. (C) Leukocyte recruitment was assessed in vivo using thioglycollate-induced peritonitis. Infiltrating cells were counted in peritoneal lavage fluid at the indicated time points (left panel) and differential counts performed at 4 hours from Wright Giemsa-stained cytospins to assess neutrophil infiltration (center panel). Representative 4-hour cytospin images are shown in the right panels.
Neutrophils from PAD4−/− mice are competent in non-NET functions. (A) Diluted, anticoagulated whole blood was incubated with 1 μM ionomycin, 100 nM PMA, or vehicle for 20 minutes at 37°C in the presence of dihydrorhodamine-123. Rhodamine+ ROS-generating neutrophils are quantified for each condition (left panel) and representative plots are shown (right two panels); n = 4-13. (B) Diluted whole blood was preincubated with 5 mM cytochalasin B for 20 minutes, then incubated with 1 μM ionomycin, 100 nM PMA, or vehicle for 10 minutes at 37°C. Lamp1+ Ly6G+ degranulated cells are quantified in the left panel, with representative plots shown in the right panels; n = 4-13. (C) Leukocyte recruitment was assessed in vivo using thioglycollate-induced peritonitis. Infiltrating cells were counted in peritoneal lavage fluid at the indicated time points (left panel) and differential counts performed at 4 hours from Wright Giemsa-stained cytospins to assess neutrophil infiltration (center panel). Representative 4-hour cytospin images are shown in the right panels.
The CLP mouse model of sepsis involves ligation of the cecum followed by perforation, allowing fecal contents to be extruded into the peritoneal cavity resulting in polymicrobial sepsis.37 In this model, circulating cell-free DNA is elevated in plasma,19 and NETs are seen in liver sinusoids.20 We performed CLP37 in PAD4-deficient animals and compared them to their PAD4+/+ and PAD4+/− littermates. We hypothesized that PAD4−/− mice would be the most susceptible in this model due to a reduced ability to kill bacteria via NETosis, especially in low-grade CLP where PAD4+/+ mice are able to contain the infection well.
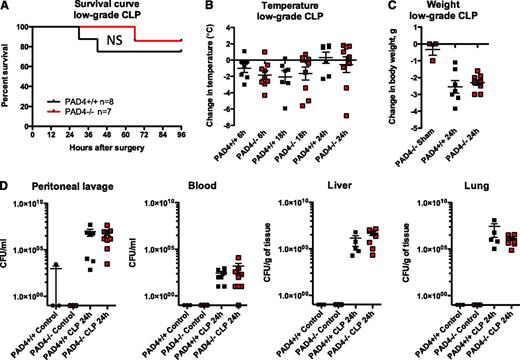
We were surprised to find that all genotypes responded similarly to low-grade CLP (Figure 2). Mice exhibited similar mortality (Figure 2A) and clinical signs of sepsis, including hypothermia (Figure 2B) and weight loss (Figure 2C). As measured in a separate group of animals, blood cell counts were not statistically different between genotypes (see supplemental Figure 1A-C on the Blood Web site). Leukocyte peritoneal recruitment occurred to a similar extent (supplemental Figure 1D-E), further demonstrating the capacity of PAD4-deficient neutrophils to transmigrate. PAD4−/− mice had lower plasma DNA levels (supplemental Figure 1F), indicating that a significant portion of circulating DNA in sepsis originates from NETs. The bacterial load was similar in blood and no difference was seen in bacterial CFUs in the peritoneum, liver, or lung in low-grade sepsis (Figure 2D). Plasma alanine aminotransferase levels were similarly elevated in both PAD4+/+ and PAD4−/− animals (supplemental Figure 1G), suggesting that NETs are not responsible for bacterial infection-induced liver injury. Importantly, we observed no increase in mortality in the PAD4−/− mice.
PAD4−/− mice are equally as susceptible to polymicrobial sepsis as their PAD4+/+ littermates in the CLP model. Mice were subjected to low-grade CLP or sham operation (Sham). (A) Survival curves indicating similar survival rates of PAD4−/− mice and their PAD4+/+ littermates in low-grade CLP. (B) Loss of body temperature was similar in PAD4−/− and PAD4+/+ mice. (C) Weight loss was comparable in PAD4+/+ vs PAD4−/− mice, although sham-operated mice lost minimal body weight. (D) Bacterial load was measured in peritoneal lavage fluid, blood, and liver and lung homogenates. Minimal CFUs were detected in control nonoperated mice. Genotypes and treatments were as indicated. No statistical differences between PAD4+/+ and PAD4−/− mice were detected. NS, not significant. PAD4+/+, black; PAD4−/−, red.
PAD4−/− mice are equally as susceptible to polymicrobial sepsis as their PAD4+/+ littermates in the CLP model. Mice were subjected to low-grade CLP or sham operation (Sham). (A) Survival curves indicating similar survival rates of PAD4−/− mice and their PAD4+/+ littermates in low-grade CLP. (B) Loss of body temperature was similar in PAD4−/− and PAD4+/+ mice. (C) Weight loss was comparable in PAD4+/+ vs PAD4−/− mice, although sham-operated mice lost minimal body weight. (D) Bacterial load was measured in peritoneal lavage fluid, blood, and liver and lung homogenates. Minimal CFUs were detected in control nonoperated mice. Genotypes and treatments were as indicated. No statistical differences between PAD4+/+ and PAD4−/− mice were detected. NS, not significant. PAD4+/+, black; PAD4−/−, red.
We next subjected mice to a severe sepsis challenge by ligating 75% of the cecum and puncturing with an 18G needle (high-grade CLP, Figure 3). Again, we saw that mice of all genotypes were similarly susceptible to death (Figure 3A). Bacterial burden was similar in the blood, liver, and lung (Figure 3B), as was the degree of hypothermia (Figure 3C). Plasma DNA was significantly higher in PAD4+/+ and PAD4+/− mice compared with PAD4−/− animals (Figure 3D). However, the levels of circulating DNA were lower than seen in other NET-generating models,24,38 possibly because NETs may be sequestered within organs. We were able to detect NETs in circulation (supplemental Figure 2A) and we saw a high number of H3Cit+ cells in 4 out of 6 PAD4+/+ mice, compared with 0 out of 5 PAD4−/− mice (supplemental Figure 2B). We measured cytokines in the plasma of animals and found no difference in IL-6 (Figure 3E) or IL-10 (Figure 3F). Although NETs form in the CLP model,19,20 we show that they don’t influence survival, bacteremia, or the overall condition of the mice because PAD4−/− mice responded similarly to PAD4+/+ mice.
PAD4−/− mice are similarly susceptible to bacteremia in polymicrobial sepsis as their PAD4+/+ littermates during high-grade CLP. Mice were subjected to high-grade CLP. (A) Survival curves indicating similar survival rates of PAD4−/− mice and their PAD4+/− or PAD4+/+ littermates in high-grade CLP. (B) Bacterial CFUs were quantified from the blood, liver, and lung 18 hours after high-grade CLP to determine systemic bacteremia and were not significantly different between genotypes. (C) Loss of body temperature was similar in PAD4−/− mice and their littermates. (D) High-grade CLP resulted in higher increases in plasma DNA in PAD4+/+ or PAD4+/− mice compared with PAD4−/− mice. (E-F) Cytokine levels in the plasma of mice. IL-6 (E) and IL-10 (F) were similarly produced in mice of all genotypes; n = 4-8. (G) Survival curve of PAD4+/+ or PAD4−/− mice that were given the broad-spectrum antibiotic imipenem/cilastatin 4 to 6 hours after CLP. Mortality was accelerated in the PAD4−/− mice (P = .043). (H) Bacterial CFUs were not statistically different between PAD4+/+ or PAD4−/− mice in the blood, liver, or lung 24 hours after CLP. NS, not significant. PAD4+/+, black; PAD4+/−, blue; and PAD4−/−, red.
PAD4−/− mice are similarly susceptible to bacteremia in polymicrobial sepsis as their PAD4+/+ littermates during high-grade CLP. Mice were subjected to high-grade CLP. (A) Survival curves indicating similar survival rates of PAD4−/− mice and their PAD4+/− or PAD4+/+ littermates in high-grade CLP. (B) Bacterial CFUs were quantified from the blood, liver, and lung 18 hours after high-grade CLP to determine systemic bacteremia and were not significantly different between genotypes. (C) Loss of body temperature was similar in PAD4−/− mice and their littermates. (D) High-grade CLP resulted in higher increases in plasma DNA in PAD4+/+ or PAD4+/− mice compared with PAD4−/− mice. (E-F) Cytokine levels in the plasma of mice. IL-6 (E) and IL-10 (F) were similarly produced in mice of all genotypes; n = 4-8. (G) Survival curve of PAD4+/+ or PAD4−/− mice that were given the broad-spectrum antibiotic imipenem/cilastatin 4 to 6 hours after CLP. Mortality was accelerated in the PAD4−/− mice (P = .043). (H) Bacterial CFUs were not statistically different between PAD4+/+ or PAD4−/− mice in the blood, liver, or lung 24 hours after CLP. NS, not significant. PAD4+/+, black; PAD4+/−, blue; and PAD4−/−, red.
In order to better understand the potential harmful effects NETs could have in this model, we repeated the high-grade CLP experiments but provided mice with antibiotics after the onset of sepsis to reduce bacterial burden. Mortality was delayed in all mice compared with those not receiving antibiotics, but we were surprised to see that after half of the mice died in both groups, PAD4−/− mice fared slightly worse than PAD4+/+ mice (Figure 3G). The PAD4−/− mice did not, however, have an increased bacterial load 24 hours after CLP (Figure 3H), so the inability to kill bacteria was not likely the cause of this small increase in mortality.
There is vast evidence that aberrant NET production has pathological consequences in noninfectious conditions, including thrombosis,28 autoimmune diseases,39,40 and ischemia/reperfusion injury.29,41 Histones are an integral part of NETs4,30 and are cytotoxic to endothelial and epithelial cells.9,35 In vivo, histones contribute substantially to mortality in sepsis9 and can induce thrombocytopenia.42 In order to study the effect of NETs and endotoxemia in mice without a live bacterial infection, we turned to established models of LPS-induced shock9,43 to generate large amounts of circulating nucleosomes in vivo. While not an ideal model of human sepsis, LPS infusion allowed us to investigate the host response leading to endotoxemic shock, which has been extensively studied experimentally.9,44 Also, we considered that while antibiotics used in patients kill pathogens, toxic products such as LPS may remain. In the LPS model, mortality is induced by the innate immune response, including cytokine storm and organ damage.
IV infusion of LPS from Salmonella enterica serotype typhimurium in PAD4+/+ mice resulted in reduced platelet and leukocyte counts (supplemental Figure 3A) and release of cell-free nucleosomes and lactate dehydrogenase, a marker of cell lysis, into circulation in mice9 (supplemental Figure 3B). Furthermore, supplemental Figure 3C shows histone release and fragmentation over time, similarly to that reported by Xu et al.9 Although necrotic cells also release nucleosomes in endotoxemia,45 we saw that with increasing DNA release over time, H3Cit, a NET biomarker, became detectable in the plasma of PAD4+/+ mice (supplemental Figure 3D), as was previously shown using low and high doses of LPS,43 indicating that NETs are formed in our model.
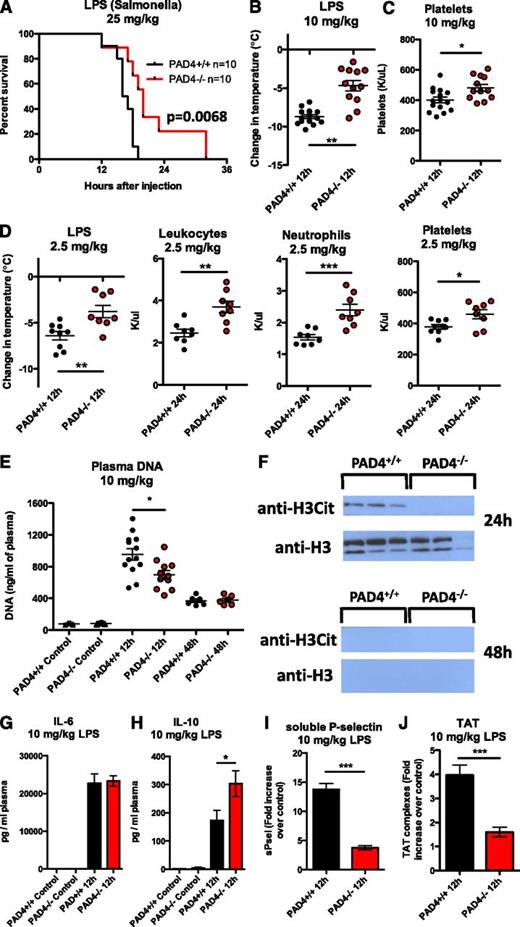
To investigate the effect of NETs in the LPS model, we compared PAD4−/− to PAD4+/+ mice. Mortality was significantly (P < .007) delayed, but not prevented, in PAD4−/− mice after a highly lethal dose of LPS (25 mg/kg; Figure 4A). An intermediate dose (10 mg/kg) resulted in 43.5% mortality in PAD4+/+ mice by 24 hours compared with 4.3% mortality in PAD4−/− mice (P < .04) (supplemental Figure 3F). PAD4−/− mice became less hypothermic (Figure 4B) and less thrombocytopenic (Figure 4C) than PAD4+/+ mice. This effect was similar using a nonlethal dose (Figure 4D and supplemental Figure 3G), along with significantly higher leukocyte and neutrophil numbers in circulation (Figure 4D). Plasma DNA levels were significantly lower in PAD4−/− mice 12 hours after LPS infusion (Figure 4E), indicating again that a portion of the nucleosomes released come from NETs. H3Cit was not detected in any PAD4−/− mouse plasma, whereas it was present in PAD4+/+ mice 24 hours after LPS injection (Figure 4F). This indicates that NETs form and that PAD4, and not another PAD family member, is responsible for the H3Cit found in circulation. By 48 hours, cell-free histone H3 and DNA levels were reduced and H3Cit was no longer detectable in plasma (Figure 4E-F). It is important to note that DNA levels were elevated in both genotypes, indicating that a substantial portion of the extracellular chromatin found in this model was coming from affected tissue rather than NETs. Neutrophils with hypercitrullinated histones were prominent in the lung 24 hours after LPS infusion in PAD4+/+ mice (supplemental Figure 3E). As we and others have previously shown, the formation of NETs in the lung is injurious and reduces lung function.46,47
PAD4−/− mice release less extracellular DNA and are better protected than PAD4+/+ mice in an LPS-induced endotoxemic shock model. (A) Mice were injected IV with a lethal dose of LPS (25 mg/kg) and monitored every 2 hours beginning at hour 12 for moribundity. (B-C) Mice injected IV with a less lethal LPS dose (10 mg/kg) were measured for signs of hypothermia and thrombocytopenia. PAD4−/− mice had a milder temperature drop (B) and had higher platelet counts than PAD4+/+ mice (C). (D) Using a sublethal dose (2.5 mg/kg), which allowed us to follow mice surviving for 24 hours, similar differences in hypothermia and thrombocytopenia were observed. Total leukocyte and neutrophil levels were also higher in PAD4−/− mice. (E-F) Plasma was collected at the time of sacrifice and analyzed for NET biomarkers. (E) Twelve hours after LPS infusion, DNA levels were lower in PAD4−/− mice. (F) Both histone H3 and H3Cit were identified in the plasma of PAD4+/+ mice at 24 hours and were no longer detected at 48 hours postinjection. H3Cit was not found in PAD4−/− mouse plasma, whereas histone H3 was detected. Representative of n = 7 (24 hours) and n = 6 (48 hours). (G-H) IL-6 levels (G) were similar in PAD4+/+ and PAD4−/− mice at 12 hours, whereas IL-10 levels (H) were higher in PAD4−/− mice. PAD4+/+, n = 13. PAD4−/− n = 12. (I-J) sPsel levels (I) and TAT complexes (J) in plasma were significantly elevated in PAD4+/+ mice compared with PAD4−/− mice, indicating that the presence of NETs activates platelets (increases P-selectin shedding) and induces the generation of thrombin. Results are expressed as fold increase over control, untreated mice. PAD4+/+, black, n = 13; PAD4−/−, red, n = 11. *P < .05, **P < .01, ***P < .001.
PAD4−/− mice release less extracellular DNA and are better protected than PAD4+/+ mice in an LPS-induced endotoxemic shock model. (A) Mice were injected IV with a lethal dose of LPS (25 mg/kg) and monitored every 2 hours beginning at hour 12 for moribundity. (B-C) Mice injected IV with a less lethal LPS dose (10 mg/kg) were measured for signs of hypothermia and thrombocytopenia. PAD4−/− mice had a milder temperature drop (B) and had higher platelet counts than PAD4+/+ mice (C). (D) Using a sublethal dose (2.5 mg/kg), which allowed us to follow mice surviving for 24 hours, similar differences in hypothermia and thrombocytopenia were observed. Total leukocyte and neutrophil levels were also higher in PAD4−/− mice. (E-F) Plasma was collected at the time of sacrifice and analyzed for NET biomarkers. (E) Twelve hours after LPS infusion, DNA levels were lower in PAD4−/− mice. (F) Both histone H3 and H3Cit were identified in the plasma of PAD4+/+ mice at 24 hours and were no longer detected at 48 hours postinjection. H3Cit was not found in PAD4−/− mouse plasma, whereas histone H3 was detected. Representative of n = 7 (24 hours) and n = 6 (48 hours). (G-H) IL-6 levels (G) were similar in PAD4+/+ and PAD4−/− mice at 12 hours, whereas IL-10 levels (H) were higher in PAD4−/− mice. PAD4+/+, n = 13. PAD4−/− n = 12. (I-J) sPsel levels (I) and TAT complexes (J) in plasma were significantly elevated in PAD4+/+ mice compared with PAD4−/− mice, indicating that the presence of NETs activates platelets (increases P-selectin shedding) and induces the generation of thrombin. Results are expressed as fold increase over control, untreated mice. PAD4+/+, black, n = 13; PAD4−/−, red, n = 11. *P < .05, **P < .01, ***P < .001.
To further investigate the effect of PAD4-deficiency in the endotoxemia model, we measured plasma cytokine levels and found similar levels of the proinflammatory cytokine IL-6 in PAD4+/+ and PAD4−/− animals (Figure 4G). Interestingly, IL-10, an anti-inflammatory cytokine that is critical for reducing the progression to irreversible shock,48 was significantly higher in PAD4−/− mice (Figure 4H). The increased IL-10 in the PAD4−/− mice may contribute to the protective phenotype seen in the LPS model. It has been reported that neutrophils are major producers of IL-10 in mouse sepsis models.49 We tested the capacity of neutrophils from PAD4+/+ or PAD4−/− mice to make IL-10 ex vivo50 and found no differences in neutrophil IL-10 production when cultured with LPS (supplemental Figure 4). Moreover, using sPsel as a biomarker of systemic platelet and/or endothelial activation, we found that PAD4+/+ mice had significantly more sPsel in circulation (Figure 4I). TAT complexes were much higher in PAD4+/+ mice compared with PAD4−/− mice (Figure 4J). The reduced inflammatory and hypercoagulable/prothrombotic state in PAD4−/− mice in response to LPS could be explained by the absence of NETs.10,11 Thus, PAD4−/− mice exhibit reduced signs of septic shock in an endotoxemia model.
Discussion
During experimental endotoxemia, large quantities of histones are released into circulation9 in complex with DNA as nucleosomes.51 The protective effects of an absence of NETs are likely found both at the organ level and systemically, resulting in reduced septic shock morbidity and mortality. It is important to note that there were still prominent levels of DNA and histones in PAD4−/− animals, likely coming from necrotic cells. However, our results indicated that the contribution by NETs was substantial enough to impact mortality by increasing the inflammatory response but that the extracellular chromatin released from tissue was still damaging since PAD4−/− mice were not fully protected. Additional global effects of PAD4-deficiency in the LPS model are possible and we cannot solely attribute the improved outcome in the PAD4−/− mice to lack of NETs. The increased anti-inflammatory response in the PAD4−/− mice is of high interest and could be due to higher numbers of surviving, non-NETing neutrophils producing IL-10. In the presence of live pathogens in the CLP model, however, the potential benefits of the reduced toxicity of NETs may be reduced/negated by the ongoing inflammatory response to the growing bacterial burden.
Our study supports previously published reports that extracellular histones are highly procoagulant, prothrombotic, and injurious when released within the vasculature, resulting in platelet-dependent thrombin generation, thrombocytopenia, endothelial cell death, and multi-organ dysfunction.9,31-33,42 We have previously shown that NETs can be dismantled by heparin23 and that heparin inhibits histone-mediated platelet aggregation.23,42 Wildhagen et al recently reported that non-anticoagulant heparin reduces histone toxicity and thus is protective in CLP and endotoxemia.52 Combined with our current results, it appears that this beneficial effect of heparin in endotoxemia may be due in part to effects on histones released as a component of NETs. Anti-histone treatments are effective at protecting mice from death only in the presence of antibiotics.9 However, clinical use of activated protein C, which among other functions, cleaves histones, is complicated by side effects.53
The genetic engineering approach of deleting PAD4 function agrees with a new report showing that use of Cl-amidine, a pan-PAD (PADs 1-4) inhibitor, in a similar CLP sepsis model, is not immunosuppressive.54 The pan-PAD inhibitor actually provides a survival advantage, not seen with the absence of NETs in our study using the PAD4−/− mice. This indicates that in addition to PAD4, other PADs may also be involved in the negative effects of bacterial sepsis. This could be due to citrullination of additional cellular and plasma proteins.
Our study using PAD4−/− mice differs from previously published work using DNase infusion17,19,22 in that NETs are not released in PAD4−/− mice. PAD4+/+ mice administered DNase may suffer from the reduced ability to perform other antimicrobial functions such as phagocytosis at infection sites once their neutrophils have formed NETs. Some cells may undergo “vital NETosis,” and retain function as anuclear cytoplasts,6,18 but their existence in polymicrobial sepsis remains unknown. Also, microbes possibly amassed in NETs, upon digestion by DNase injection, may become rapidly liberated and this could increase their dissemination.18 However, our results indicate that bacteremia is not affected by PAD4-deficiency in the CLP model. The PAD4−/− mice had slightly worse outcome than PAD4+/+ mice in the presence of antibiotics, but this was not due to increased bacterial burden. Under these conditions, NETs may have a positive feedback role at later time points. Alternatively, NETs may be involved in the sequestration of dead bacteria and their clearance by macrophages. NETs may be more critical for controlling larger pathogens such as fungal hyphae or large bacterial aggregates.55 Indeed, the extent of the physiological role of NETs in the direct killing of bacteria remains elusive.6,56 This is further supported by a recent report of a patient with Papillon-Lefevre syndrome lacking serine proteases and the ability to make NETs, but not exhibiting a marked immunodeficiency.57
PAD4-deficient mice have provided specific evidence of neutrophil antimicrobial activity in the skin.26 When using a mutant, DNase-null bacteria, the lesion size was reduced in WT but not PAD4−/− mice, providing in vivo evidence of the ability of NETs to kill bacteria.26 It is, however, important to note that the use of a WT, nuclease-secreting microbe did not result in significantly larger skin lesions or higher bacterial CFUs in PAD4-deficient animals in the model of necrotizing fasciitis.26 We show here that PAD4-deficiency has no impact on bacteremia in polymicrobial sepsis. In this model, NETs appear not to be involved in destroying or sequestering live microbes from the blood. Since NETs are not sufficient to clear bacterial infection, they may indeed cause more harm than good. Moreover, because many bacteria are able to evade NETs by secreting nucleases,26,58-60 the actual physiological impact of the antibacterial function of NETs may be minor, as seen in our study.
Our results indicate that blood infections are not exacerbated in the absence of NETs and that excessive NET production contributes to the pathology of nucleosome-induced mortality. We did not see an improvement in the PAD4−/− mice in CLP, suggesting that NET inhibition would not be beneficial in bacterial sepsis. However, it may be of interest in the context of sudden inflammatory response syndrome where an infection is not the original cause. Indeed, our results have important implications for future development of NET-targeted therapeutics. We have previously proposed that inhibiting PAD4 could be highly beneficial in the context of thrombosis11 and ischemia/reperfusion injury.29 We now show that this would not likely result in a drastic immunosuppressive effect. Under PAD4 inhibition, neutrophils would be able to reach areas of infection and have antimicrobial effects other than by forming NETs, and the cytotoxic impact of NETs on the host would be eliminated. Although being investigated,61 no specific PAD4 inhibitors are yet available and there is reluctance to develop them due to fear of infection. We hope that our studies, showing that PAD4 inhibition would not result in a severely immunocompromised host, will encourage such development.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Wolfgang G. Junger, Tanya N. Mayadas, and Ulrich H. von Andrian for helpful discussions, and Lesley Cowan for assistance with the preparation of this manuscript.
This study was supported by grants from the National Institutes of Health National Heart, Lung, and Blood Institute (R01HL102101) (D.D.W.) and the National Cancer Institute (R01CA136856) (Y.W.).
Authorship
Contribution: K.M. designed and performed experiments, analyzed data, and wrote the paper; T.A.F., N.L.Z., S.L.W., M.D., and M.G. performed experiments and analyzed data; Y.W. provided the PAD4−/− mice, helpful advice, and contributed to discussions; and D.D.W. supervised the study, designed experiments, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for T.A.F. is University Medical Center Hamburg-Eppendorf, Institute of Clinical Chemistry and Laboratory Medicine, Hamburg, Germany.
Correspondence: Denisa D. Wagner, Program in Cellular and Molecular Medicine, Boston Children’s Hospital, 3 Blackfan Circle, 3rd Floor, Boston, MA 02115; e-mail: denisa.wagner@childrens.harvard.edu.