Key Points
Chemotherapy and radiotherapy deplete ILCs from the blood; ILC reconstitution after allogeneic HSCT is slow.
High frequencies of activated ILCs with tissue homing potential before allogeneic HSCT are associated with reduced risk for GVHD.
Allogeneic hematopoietic stem cell transplantation (HSCT) is widely used to treat hematopoietic cell disorders but is often complicated by graft-versus-host disease (GVHD), which causes severe epithelial damage. Here we have investigated longitudinally the effects of induction chemotherapy, conditioning radiochemotherapy, and allogeneic HSCT on composition, phenotype, and recovery of circulating innate lymphoid cells (ILCs) in 51 acute leukemia patients. We found that reconstitution of ILC1, ILC2, and NCR−ILC3 was slow compared with that of neutrophils and monocytes. NCR+ ILC3 cells, which are not present in the circulation of healthy persons, appeared both after induction chemotherapy and after allogeneic HSCT. Circulating patient ILCs before transplantation, as well as donor ILCs after transplantation, expressed activation (CD69), proliferation (Ki-67), and tissue homing markers for gut (α4β7, CCR6) and skin (CCR10 and CLA). The proportion of ILCs expressing these markers was associated with a decreased susceptibility to therapy-induced mucositis and acute GVHD. Taken together, these data suggest that ILC recovery and treatment-related tissue damage are interrelated and affect the development of GVHD.
Introduction
Allogeneic hematopoietic stem cell transplantation (HSCT) can be a life-saving treatment of patients with hematologic malignancies. Although curative for many, it is characterized by significant morbidity and mortality. Preparative regimens including chemotherapy and radiotherapy can cause serious harm to mucosal tissues by inducing apoptosis of rapidly dividing epithelial cells.1 In current models for the development of acute graft-versus-host disease (GVHD), such tissue destruction plays an important role in the initiation phase of a process that, through activation of innate immune responses, eventually leads to donor T-cell activation and immune-mediated tissue damage.2,3
Innate lymphoid cells (ILCs) comprise a family of innate cells with a lymphoid morphology that lack rearranged antigen receptors. ILCs play key roles in the early stages of the immune response and are important effectors in tissue remodeling and repair.4,5 ILCs can be divided into functionally different groups, based on the expression of specific transcription factors and signature cytokines.6,-8 Group 1 ILCs are characterized by the expression of T-bet and interferon (IFN)-γ.9 ILC1 are activated through IL-12 and/ or IL-18 and are increased in the gut of patients with Crohn colitis.9,10 Group 2 ILCs depend on the transcription factors GATA311,12 and RORα13,14 and produce the type 2 cytokines IL-13 and IL-5 upon activation by thymic stromal lymphopoietin (TSLP), IL-33, and/or IL-25.15 ILC2 are important in protecting the airway epithelium against the cytopathic effects of respiratory viruses,5 but they also play a role in pathological responses such as (atopic) dermatitis,16,17 chronic rhinosinusitis,15 and liver fibrosis.18 Group 3 ILCs represent all RORγt-dependent ILCs, including lymphoid tissue–inducer (LTi) cells, natural cytotoxicity receptor (NCR)-negative ILC3, and NCR+ ILC3, and are activated upon stimulation with IL-1β and IL-23.19,-21 NCR+ ILC3 are an important innate source of IL-22, a potent cytokine that acts directly on epithelial cells to induce proliferation, survival, and repair.22,23 Recently, it was demonstrated in a mouse model of acute intestinal GVHD that sublethal radiotherapy increased intestinal IL-22 production by IL-23 responsive, radiotherapy-resistant host ILCs. Subsequent allogeneic HSCT in IL-22–deficient recipients led to severe GVHD, causing significantly increased tissue damage, with apoptosis of crypt cells and loss of epithelial integrity, and increased GVHD-related mortality.24 These data suggest that IL-22–producing NCR+ ILC3 may dampen the detrimental effects of GVHD, at least in the intestine of mice. Whether ILC3 have similar protective effects in human GVHD is unknown.
We longitudinally studied loss and recovery of groups 1, 2, and 3 ILCs in the peripheral blood of adult patients with acute leukemia, after induction chemotherapy and after allogeneic HSCT. ILCs were lost upon induction and conditioning chemotherapy, and their recovery after allogeneic HSCT was slow. Reconstituting ILCs were of donor origin. Expression of CD69 on ILCs in patients before allogeneic HSCT (after induction chemotherapy) and appearance of donor NCR+ ILC3 after HSCT correlated with a decreased incidence of GVHD, which may suggest that these cells have protective effects in the context of treatment-related tissue damage.
Materials and methods
Study participants and clinical protocols
Study protocols were approved by the Medical Ethical Committees of the Karolinska Institutet, Stockholm, Sweden, and of the Academic Medical Center, Amsterdam, The Netherlands. All participants signed informed consent in accordance with the Declaration of Helsinki. We performed a pilot study with freshly isolated peripheral blood mononuclear cells (PBMCs) of healthy controls (n = 10) and of patients with acute myeloid leukemia (AML) (n = 9) or acute lymphoblastic leukemia (ALL) (n = 2), treated at the Academic Medical Center in Amsterdam (Table 1). The median ages of healthy persons (36 years, range 30-56) and patients (50 years, range 19-70) were not significantly different. Patient material was collected at consecutive time points before, during, and after 2 cycles of induction chemotherapy, after conditioning therapy at the day of allogeneic HSCT, and at 4-week intervals after allogeneic HSCT. To confirm and extend the findings obtained from this small cohort, we included patients from a second, larger and independent cohort. At the Karolinska Institutet, a large allogeneic HSCT biobank for patients receiving allogeneic HSCT was set up between June 2005 and December 2010. Clinical data were collected prospectively. From all patients with AML (n = 67) in this biobank, 40 patients were randomly selected based on availability of PBMCs at 2 time points: (1) collected at the day conditioning therapy was started (after completing (re-)induction chemotherapy, before allogeneic HSCT), and (2) at 12 weeks after allogeneic HSCT (median, day 84 after allogeneic HSCT; range day 56-day 112; of 6 patients, no sample was available at day 84 and the nearest day at which material was available was chosen). Most patients had AML (n = 37), and three had myelodysplastic syndrome–refractory anemia with excess blasts–2; GVHD did not develop in 12 of the patients, and acute GVHD grade I-IV did develop in 28 patients (Table 1; no significant age differences between GVHD+/GVHD– groups). The majority of patients had a clinical follow-up of >12 months (n = 37). Diagnosis of cutaneous GVHD was made on the basis of clinical considerations and was not always confirmed by biopsy of affected tissue; gut GVHD was always confirmed by histopathology. From September 2007 onward, mucositis grading was evaluated regularly by a dentist, according to the World Health Organization Toxicity Grading Scale (daily from the day of admission) and the Oral Mucositis Assessment Scale (3× per week). For comparison, PBMCs of healthy individuals were cryopreserved before analysis (n = 8; median age 29 years, range 26-32; P < .05 compared with patients in cohort 2).
Isolation of cells
PBMCs were obtained from whole blood by Ficoll-Paque (GE Healthcare) density gradient centrifugation. For cryopreservation, PBMCs were resuspended in cryoprotective media containing 10% dimethyl sulfoxide and fetal bovine serum. Before flow cytometry analysis, the PBMCs were rapidly thawed.
Flow cytometry analysis
The following antibodies to human proteins were used: fluorescein isothiocyanate (FITC)-conjugated anti-CD1a (HI149), anti-CD11c (3.9), anti-CD14 (HCD14), anti-CD19 (HIB19), anti-CD34 (581), anti-CD94 (DX22), anti-CD123 (6H6), anti-FcER1α (AER-37), phycoerythrin (PE)–conjugated anti-CCR10 (6588-5), Pacific Blue–conjugated anti-CLA (HECA-452), allophycocyanin-indotricarbocyanine (APC-Cy7)–conjugated anti-CD25 (BC96), Brilliant Violet 570–conjugated anti-CD56 (HCD56), Brilliant Violet 605–conjugated anti-CCR6 (G034E3), Brilliant Violet 650–conjugated anti-α4β7 (Act-1; the α4β7 monoclonal antibody was obtained through the National Institutes of Health AIDS Reagent Program, Division of AIDS, National Institute of Allergy and Infectious Diseases [cat#11718] from Dr A.A. Ansari and subsequently biotinylated using the FluoReporter Mini-Biotin-XX Protein Labeling Kit from Invitrogen), Alexa Fluor 700–conjugated anti-CD69 (FN50; all from BioLegend); FITC-conjugated anti-TCRαβ (IP26), anti-TCRγδ (B1), Alexa Fluor 647–conjugated anti-CRTH2 (CD294; BM16), V500-conjugated anti-CD3 (UCHT1; all from BD Biosciences); R Phycoerythrin-Cyanine 5 (PE-Cy5)–conjugated NKp44 (Z231), R Phycoerythrin-Cyanine 5.5 (PE-Cy5.5)–conjugated anti-CD117 (104D2D1), R Phycoerythrin-Cyanine 7 (PE-Cy7)–conjugated anti-CD127 (R34.34; both from Beckman Coulter), and FITC-conjugated anti-BDCA2 (CD303; AC144; Milenyi Biotec). Intracellular staining for Ki-67 and GATA3 was done using Brilliant Violet 711–conjugated anti–Ki-67 (Ki67; BioLegend), PE-conjugated anti-GATA3 (TWAJ, eBioscience), and the Foxp3 permeablilization/fixation kit according to the manufacturer’s instructions.
For phenotypic analyses by flow cytometry, data were collected with an LSR-Fortessa instrument (BD Biosciences) and analyzed with FlowJo software (TreeStar). For chimerism analysis, Lin−CD127+ ILCs from peripheral blood were sorted on a FACSAria (BD Biosciences).
Chimerism analysis
Genomic DNA of sort-purified Lin−CD127+ ILCs was isolated with the QIAamp DNA Micro Kit (Qiagen) according to the manufacturer’s protocol. Donor-vs-patient chimerism was determined by analyzing genomic polymorphisms through profiling of short tandem repeat (STR) DNA loci using the PowerPlex 16 System (Promega Corporation, Madison, WI). Amplified fragments were separated by capillary electrophoresis conducted on the ABI PRISM 3130 XL Genetic Analyzer (Applied Biosystems), and corresponding peak area values were transferred to the Applied Biosystems GeneMapper 4.1 (Applied Biosystems) for evaluation.
Statistical analysis
Data are shown as median values with interquartile ranges. Differences between healthy controls and patients were calculated using the 2-tailed Mann-Whitney U test, using Graphpad Prism 5 for Mac OS X. For comparison of separate time points, the Wilcoxon signed rank test was used. Survival curves were analyzed by log-rank tests. P values <.05 were considered significant.
Results
Differential recovery of peripheral blood ILC subsets after induction chemotherapy and after allogeneic HSCT
Circulating ILCs are phenotypically identified by a bright expression of the α-chain of the IL-7 receptor (CD127) in the absence of lineage markers for T cells (CD3, TCR), B cells (CD19), natural killer (NK) cells (CD94), myeloid and plasmacytoid dendritic cells (CD1a, CD11c, CD123, BDCA2), monocytes and macrophages (CD14), mast cells (FcεR1), and stem cells (CD34; Figure 1A).15 ILC subsets are distinguished based on the expression of CRTH2 (chemoattractant receptor–homologous molecule expressed on T-helper 2 lymphocytes; CD294), CD117 (c-Kit), and the activating natural cytotoxicity receptor (NCR) NKp44. Group 1 ILCs are CRTH2−CD117−NKp44− ILCs, whereas group 2 ILCs express CRTH2 with or without CD117. Group 3 ILCs are CRTH2−CD117+ ILCs that, based on expression of NKp44, can be separated into NCR+ ILC3 and NCR− ILC3.7 In healthy individuals, CRTH2+ ILCs make up the most prominent ILC subset in the peripheral blood, whereas NCR+ ILC3 are virtually absent under homeostatic conditions (Figure 1A). The cytokine expression profile of ILCs derived from the peripheral blood is similar to that of tissue-derived ILC, with ILC1 producing IFNγ (supplemental Figure 1, available on the Blood Web site), ILC2 producing type 2 cytokines like IL-13,12,15 NCR– ILC3 capable to secrete some IFNγ and IL-22 (supplemental Figure 1), and NCR+ ILC3 producing IL-22.25
Loss and recovery of ILCs after AML induction chemotherapy and allogeneic HSCT. (A) Gating strategy for the identification of peripheral blood ILCs, here shown for a healthy individual. ILCs are Lin− (CD1a−CD11c−CD14−CD19−CD34−CD123−TCRαβ−TCRγδ− BDCA2−FcεR1−CD94−), CD3− lymphocytes that are CD127+. Groups 1, 2, and 3 ILCs are identified by the expression of CRTH2, CD117, and NKp44. Numbers in quadrants indicate the percent cells in each throughout. (B) Representative dot plot of a peripheral blood sample obtained from a patient with AML, 18 days after the first-induction chemotherapy cycle. (C) Loss and recovery dynamics of total ILCs (Lin−CD127+) during 2 AML induction chemotherapy cycles (left panel, n = 11 [cohort 1, Table 1]) and after reduced-intensity conditioning allogeneic HSCT (right panel, n = 6 [cohort 1, Table 1]). Samples were taken during neutropenia (at day 21 of each induction chemotherapy cycle), during the recovery phases of the first and second induction chemotherapy cycles, and at the days of resubmission for the second (“cycle 2”) and third (“cycle 3”; good-risk AML patients only) induction chemotherapy cycles. Patients with intermediate or poor-risk AML proceeded to allogeneic HSCT after the second induction chemotherapy cycle, and samples were collected from these patients at the day of admission to receive pre-allogeneic HSCT conditioning therapy (“allo-HSCT”). Arrows with “cycle 1,” “cycle 2,” or “cycle 3/allo-HSCT” (left panel) indicate the first day of induction chemotherapy cycles or the first day of conditioning therapy preceding allogeneic HSCT; peripheral blood neutrophil numbers had completely recovered at these time points. Dashed lines represent the 95% confidence interval (CI) as measured for healthy, age-matched controls (n = 10). (D) The recovery dynamics of total ILC (Lin−CD127+), ILC1, ILC2, NCR− ILC3, and NCR+ ILC3 after induction chemotherapy (white bars) and 12 weeks after allogeneic HSCT (gray bars, n = 40 [cohort 2, Table 1]). Data in (C) and (D) are depicted as median values with interquartile ranges. *P < .05, **P < .01, ***P < .001. HC, healthy controls (black bars, n = 8).
Loss and recovery of ILCs after AML induction chemotherapy and allogeneic HSCT. (A) Gating strategy for the identification of peripheral blood ILCs, here shown for a healthy individual. ILCs are Lin− (CD1a−CD11c−CD14−CD19−CD34−CD123−TCRαβ−TCRγδ− BDCA2−FcεR1−CD94−), CD3− lymphocytes that are CD127+. Groups 1, 2, and 3 ILCs are identified by the expression of CRTH2, CD117, and NKp44. Numbers in quadrants indicate the percent cells in each throughout. (B) Representative dot plot of a peripheral blood sample obtained from a patient with AML, 18 days after the first-induction chemotherapy cycle. (C) Loss and recovery dynamics of total ILCs (Lin−CD127+) during 2 AML induction chemotherapy cycles (left panel, n = 11 [cohort 1, Table 1]) and after reduced-intensity conditioning allogeneic HSCT (right panel, n = 6 [cohort 1, Table 1]). Samples were taken during neutropenia (at day 21 of each induction chemotherapy cycle), during the recovery phases of the first and second induction chemotherapy cycles, and at the days of resubmission for the second (“cycle 2”) and third (“cycle 3”; good-risk AML patients only) induction chemotherapy cycles. Patients with intermediate or poor-risk AML proceeded to allogeneic HSCT after the second induction chemotherapy cycle, and samples were collected from these patients at the day of admission to receive pre-allogeneic HSCT conditioning therapy (“allo-HSCT”). Arrows with “cycle 1,” “cycle 2,” or “cycle 3/allo-HSCT” (left panel) indicate the first day of induction chemotherapy cycles or the first day of conditioning therapy preceding allogeneic HSCT; peripheral blood neutrophil numbers had completely recovered at these time points. Dashed lines represent the 95% confidence interval (CI) as measured for healthy, age-matched controls (n = 10). (D) The recovery dynamics of total ILC (Lin−CD127+), ILC1, ILC2, NCR− ILC3, and NCR+ ILC3 after induction chemotherapy (white bars) and 12 weeks after allogeneic HSCT (gray bars, n = 40 [cohort 2, Table 1]). Data in (C) and (D) are depicted as median values with interquartile ranges. *P < .05, **P < .01, ***P < .001. HC, healthy controls (black bars, n = 8).
To study the loss and recovery dynamics of ILC during chemotherapy and after allogeneic HSCT, we longitudinally measured the numbers of circulating ILCs in patients with acute leukemia (cohort 1, Table 1). We did not measure peripheral blood ILC numbers at the time of diagnosis, because AML frequently causes a decrease in normal hematopoietic cells including ILCs. By inference, low ILC numbers after the first-induction chemotherapy cycle may be AML-induced rather than chemotherapy-related. The second AML induction chemotherapy cycle and the preallogeneic HSCT conditioning chemoradiotherapy decimated the number of circulating ILCs (Figure 1B-C). Recovery of ILCs was incomplete 6 months after allogeneic HSCT (Figure 1C).
To confirm and extend these findings, we measured the number of circulating ILCs in a larger cohort of 40 patients with AML (cohort 2, Table 1). Samples were taken at 2 consecutive time points: (1) after completing AML (re-)induction chemotherapy (before allogeneic HSCT) and (2) at 12 weeks after allogeneic HSCT. At the first time point, after completing (re-)induction chemotherapy, peripheral blood neutrophil and monocyte counts had fully recovered (supplemental Figure 2, white bars). However, the number of ILC2 was strongly decreased compared with control values, and this decrease was responsible for the lower number of total circulating ILCs (Figure 1D, white bars). In contrast, the number of circulating NCR+ILC3 was modestly but significantly increased compared with reference values. Numbers of ILC1 and NCR− ILC3 tended to be lower but were not significantly different from healthy individuals (Figure 1D, white bars). At the second time point, 12 weeks after allogeneic HSCT, numbers of monocytes, neutrophils, and the total pool of lymphocytes (including T cells, B cells, NK cells, and ILCs) were not statistically different from healthy control reference values (supplemental Figure 2, gray bars). Numbers of circulating ILC2 were strongly decreased, whereas ILC1 and NCR− ILC3 were more modestly but significantly lower than normal, and a modest but significant increase of NCR+ ILC3 was again observed (Figure 1D, gray bars). Notably, ILC2 in our study are defined by the expression of CRTH2. Because it cannot be excluded that reconstituting ILC2 lack or downregulated CRTH2, we tested 4 allogeneic HSCT recipients for the presence of CRTH2− ILC2 (which do express ST2 and/or GATA3), but these cells could not be detected (supplemental Figure 3). Finally, we correlated peripheral blood numbers of ILC with cyclosporine and steroid dose and found no significant correlations (supplemental Figure 4).
Reconstituting ILCs are activated and express markers associated with tissue homing
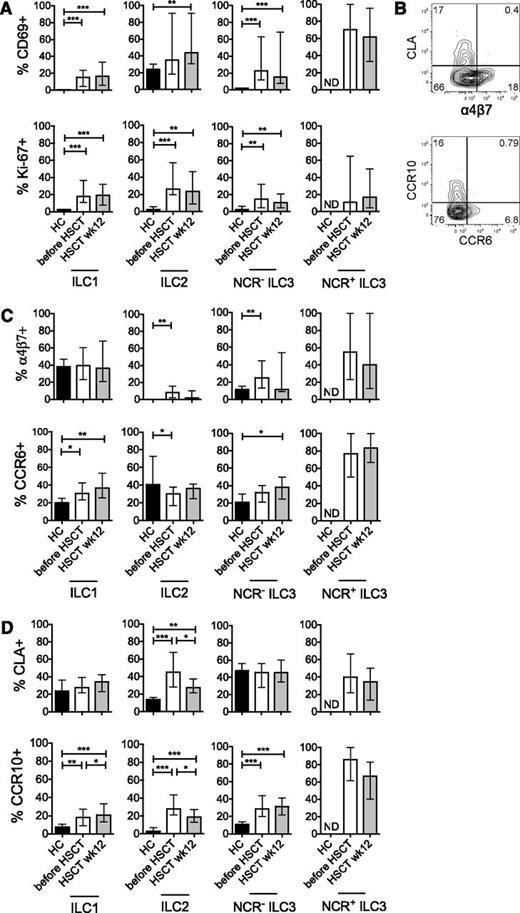
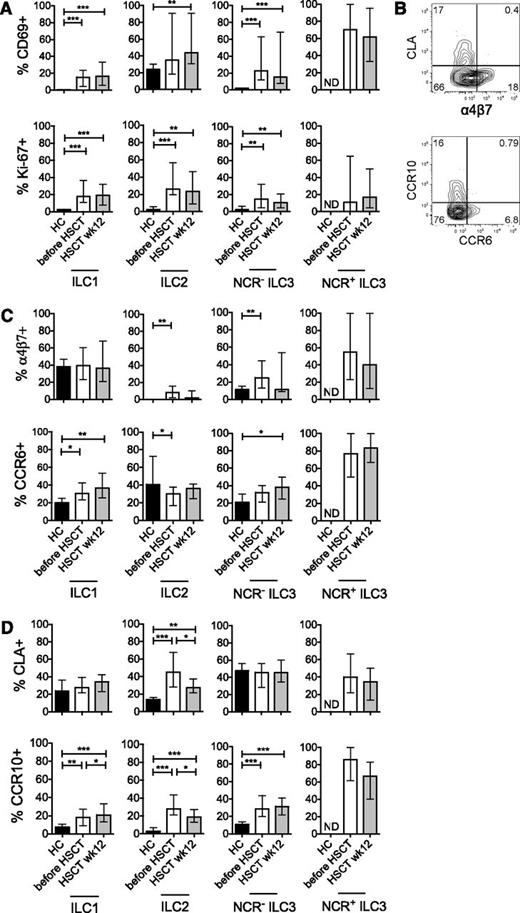
To further characterize the properties of ILC reconstituting after induction chemotherapy and allogeneic HSCT, we investigated the expression of markers associated with tissue homing, activation status, and proliferative activity. Reconstituting ILCs showed increased expression of the activation marker CD69 and the proliferation antigen Ki-67, both after induction chemotherapy (before HSCT) and when measured 12 weeks after HSCT (Figure 2A). Expression of tissue-homing markers by ILC was mutually exclusive: skin-homing markers (CLA, CCR10) expressing ILC did not express gut-homing markers, and gut-homing markers (α4β7, CCR6) expressing ILC did not express skin-homing markers (Figure 2B).26,,-29 Induction chemotherapy led to an increased expression of the gut-homing markers α4β7 (on ILC2 and NCR− ILC3) and CCR6 (on ILC1). After allogeneic HSCT, CCR6 expression was significantly increased on ILC1, as well as on NCR− ILC3 (Figure 2C). Induction chemotherapy also induced expression of the skin-homing markers CLA (by ILC2) and CCR10 (by all ILC subsets; Figure 2D). After allogeneic HSCT, all ILC subsets again showed increased CCR10 expression. The proportion of CLA+ ILC2 had decreased compared with pre-allogeneic HSCT values but was significantly increased compared with healthy controls (Figure 2D). In healthy individuals, the number of circulating NCR+ ILC3 was too low to allow analysis of their expression of activation and homing markers. However, the majority of NCR+ ILC3 that appeared in the circulation after induction chemotherapy and after allogeneic HSCT were activated and expressed gut- or skin-homing markers (Figure 2A,C-D). From these data, we concluded that reconstituting ILCs have an activated phenotype and an enhanced expression of tissue-homing markers compared with healthy individuals.
Reconstituting ILCs are activated and express markers associated with tissue homing. (A) Expression of the early activation marker CD69 (upper panels) and the proliferation antigen Ki-67 (lower panels) after induction chemotherapy (before HSCT, white bars) and 12 weeks after allogeneic HSCT (gray bars). (B) Expression of molecules associated with homing to gut (α4β7 and CCR6) or skin (CCR10 and CLA) was mutually exclusive. A representative example of homing marker expression on Lin− CD127+ ILC is depicted. (C) Expression of α4β7 (upper panels) and CCR6 (lower panels) after induction chemotherapy (before HSCT, white bars) and 12 weeks after allogeneic HSCT (gray bars). (D) Expression of CCR10 (upper panels) and CLA (lower panels) after induction chemotherapy (before HSCT, white bars) and 12 weeks after allogeneic HSCT (gray bars). Data are shown as median values with interquartile ranges. *P < .05, **P < .01, ***P < .001. HC, healthy controls (black bars, n = 8). Patient data are taken from cohort 2, Table 1 (n = 40). ND, not determined.
Reconstituting ILCs are activated and express markers associated with tissue homing. (A) Expression of the early activation marker CD69 (upper panels) and the proliferation antigen Ki-67 (lower panels) after induction chemotherapy (before HSCT, white bars) and 12 weeks after allogeneic HSCT (gray bars). (B) Expression of molecules associated with homing to gut (α4β7 and CCR6) or skin (CCR10 and CLA) was mutually exclusive. A representative example of homing marker expression on Lin− CD127+ ILC is depicted. (C) Expression of α4β7 (upper panels) and CCR6 (lower panels) after induction chemotherapy (before HSCT, white bars) and 12 weeks after allogeneic HSCT (gray bars). (D) Expression of CCR10 (upper panels) and CLA (lower panels) after induction chemotherapy (before HSCT, white bars) and 12 weeks after allogeneic HSCT (gray bars). Data are shown as median values with interquartile ranges. *P < .05, **P < .01, ***P < .001. HC, healthy controls (black bars, n = 8). Patient data are taken from cohort 2, Table 1 (n = 40). ND, not determined.
Appearance of α4β7+ CD69+ ILCs after induction chemotherapy is associated with a lower incidence of GVHD
When analyzing the level of expression of the activation marker CD69 on the ILCs of patients who recovered from induction chemotherapy but were not yet transplanted with HSC, we observed a striking dichotomy of expression of CD69. Two groups of patients were observed, with either high or low proportions of CD69+ ILCs (Figure 3A), and this correlated with the development of GVHD (Figure 3B). The majority of the patients with high proportions of CD69+ ILCs before allogeneic HSCT did not develop acute GVHD (Figure 3C). Patients with higher proportions of CD69+ ILCs also had less severe mucositis (supplemental Figure 5). Patients with high proportions of CD69+ ILCs showed a significantly higher expression of the gut-homing marker α4β7 than patients with lower proportions of CD69+ ILCs, in particular in the ILC2 and NCR− ILC3 subsets (Figure 3D); and in patients with increased expression of α4β7 on ILC2 and ILC3 after induction chemotherapy (before allogeneic HSCT), there was a lower incidence of intestinal GVHD (Figure 3E).
Appearance of α4β7+ CD69+ ILCs after induction chemotherapy is associated with a lower incidence of GVHD. (A) CD69 expression separated patients into 2 distinct groups: patients with a high or low proportion of CD69+ ILCs, in particular for ILC2, NCR− ILC3, and NCR+ ILC3. (B) Patients who did not develop acute GVHD had significantly higher proportions of CD69+ ILC before allogeneic HSCT. (C) Kaplan-Meier curves for patients who did or did not develop acute GVHD, stratified according to the expression of CD69 by ILC before HSCT. CD69hi denotes patients with a proportion of CD69+ ILC higher than median;CD69lo patients with CD69+ ILC proportions lower than median (see [A] for individual data). (D) Expression of the gut-homing molecule α4β7 by the ILCs of patients with high or low proportions of CD69+ before allogeneic HSCT. (E) Patients in whom acute GVHD of the gut did not develop had higher proportions of α4β7+ ILC2 and ILC3 before allogeneic HSCT. Squares in (A) denote healthy persons, triangles HSCT recipients (before allogeneic HSCT). Data (n = 40 [cohort 2, Table 1]) in (B,D-E) are shown as median values with interquartile ranges. All samples were taken before allogeneic HSCT. *P < .05, **P < .01, ***P < .001. HC, healthy controls (black bars, n = 8). ND, not determined.
Appearance of α4β7+ CD69+ ILCs after induction chemotherapy is associated with a lower incidence of GVHD. (A) CD69 expression separated patients into 2 distinct groups: patients with a high or low proportion of CD69+ ILCs, in particular for ILC2, NCR− ILC3, and NCR+ ILC3. (B) Patients who did not develop acute GVHD had significantly higher proportions of CD69+ ILC before allogeneic HSCT. (C) Kaplan-Meier curves for patients who did or did not develop acute GVHD, stratified according to the expression of CD69 by ILC before HSCT. CD69hi denotes patients with a proportion of CD69+ ILC higher than median;CD69lo patients with CD69+ ILC proportions lower than median (see [A] for individual data). (D) Expression of the gut-homing molecule α4β7 by the ILCs of patients with high or low proportions of CD69+ before allogeneic HSCT. (E) Patients in whom acute GVHD of the gut did not develop had higher proportions of α4β7+ ILC2 and ILC3 before allogeneic HSCT. Squares in (A) denote healthy persons, triangles HSCT recipients (before allogeneic HSCT). Data (n = 40 [cohort 2, Table 1]) in (B,D-E) are shown as median values with interquartile ranges. All samples were taken before allogeneic HSCT. *P < .05, **P < .01, ***P < .001. HC, healthy controls (black bars, n = 8). ND, not determined.
Elevated numbers of circulating NCR+ ILC3 after HSCT in patients without GVHD
Although circulating numbers of NCR+ ILC3 are nearly absent in the peripheral blood of healthy individuals, NCR+ ILC3 are readily detectable in allogeneic HSCT recipients, albeit with a large variation (Figure 1D, gray bars). A large proportion of these NCR+ ILC3 express tissue-homing markers such as α4β7, CCR6, CLA, and CCR10 (Figure 2). NCR+ ILC3 produce IL-22, a cytokine that acts on epithelial cells to restore tissue damage.30,-32 Epithelial damage induced by conditioning chemotherapy and radiotherapy has been recognized as a risk factor for developing GVHD, an observation that could be confirmed in our patients: acute GVHD developed in more patients with grade III mucositis than in patients with grade I or II mucositis (Figure 4A).33,34 Notably, 12 weeks after allogeneic HSCT, the number of circulating NCR+ ILC3 was highest in patients who had not developed acute GVHD at that time and who would not develop chronic GVHD in the ensuing months (Figure 4B). This dichotomy between high and low ILC numbers had also been observed in the first cohort and was apparent as early as 6 weeks after allogeneic HSCT (Figure 4C), a time when all patients were receiving immunosuppressive therapy to prevent GVHD, and when none of them had yet developed GVHD.
Elevated numbers of circulating NCR+ ILC3 after HSCT in patients without GVHD. (A) Mucositis was scored daily during HSCT conditioning therapy and thereafter in 24 patients (enrolled in cohort 2 after the year 2007; Table 1). Most patients in whom acute GVHD developed had grade II or grade III mucositis during conditioning therapy, whereas most patients in whom acute GVHD did not develop had grade I or grade II mucositis. (B) NCR+ ILC3 numbers 12 weeks after allogeneic HSCT for patients with or without acute GVHD or chronic GVHD (n = 40 [cohort 2, Table 1]). (C) The dichotomy between circulating NCR+ ILC3 numbers for patients in whom chronic GVHD would and would not develop was apparent as early as 6 weeks after allogeneic HSCT, a time point at which acute GVHD had developed in none of the patients and all patients were on maximal immunosuppressive therapy to prevent GVHD (n = 6 [cohort 1, Table 1]). Data are shown as median values with interquartile ranges. *P < .05, **P < .01, ***P < .001. HC, healthy controls (n = 8 [cohort 2] in panel A and n = 10 [cohort 1] in panel B).
Elevated numbers of circulating NCR+ ILC3 after HSCT in patients without GVHD. (A) Mucositis was scored daily during HSCT conditioning therapy and thereafter in 24 patients (enrolled in cohort 2 after the year 2007; Table 1). Most patients in whom acute GVHD developed had grade II or grade III mucositis during conditioning therapy, whereas most patients in whom acute GVHD did not develop had grade I or grade II mucositis. (B) NCR+ ILC3 numbers 12 weeks after allogeneic HSCT for patients with or without acute GVHD or chronic GVHD (n = 40 [cohort 2, Table 1]). (C) The dichotomy between circulating NCR+ ILC3 numbers for patients in whom chronic GVHD would and would not develop was apparent as early as 6 weeks after allogeneic HSCT, a time point at which acute GVHD had developed in none of the patients and all patients were on maximal immunosuppressive therapy to prevent GVHD (n = 6 [cohort 1, Table 1]). Data are shown as median values with interquartile ranges. *P < .05, **P < .01, ***P < .001. HC, healthy controls (n = 8 [cohort 2] in panel A and n = 10 [cohort 1] in panel B).
Tissue-homing marker expression on ILCs after allogeneic HSCT
We then investigated homing marker expression in relation to the development of GVHD after patients had received allogeneic HSCT. Twelve weeks after allogeneic HSCT, patients with high proportions of CD69+ ILC2 and NCR– ILC3 had increased expression of the gut-homing marker α4β7 compared with those with low proportions of CD69+ ILCs (Figure 5A). The proportion of α4β7+ ILC2 and α4β7+ NCR– ILC3 tended to be increased in patients in whom GVHD of the gut did not develop, but this was not significant for NCR– ILC3 (Figure 5B). Similarly, the proportion of skin-homing CLA+ ILC1 and CLA+ NCR– ILC3 were modestly but significantly increased in patients without GVHD of the skin, compared with patients with GVHD of the skin (Figure 5C). In addition, the proportion of CCR10+ ILC1 and CCR10+ NCR– ILC3 was increased in patients who did not develop cutaneous GVHD compared with healthy individuals (Figure 5C).
Tissue-homing marker expression after allogeneic HSCT. (A) Expression of the gut-homing marker α4β7 by the ILCs of patients with high or low proportions of CD69+ ILCs, 12 weeks after allogeneic HSCT. (B) Expression of the gut-homing marker α4β7 in healthy controls (black bars) and patients with and without acute GVHD of the gut. (C) Proportion of CLA+ (left panels) and CCR10+ (right panels) ILC1 and NCR– ILC3 in healthy controls (black bars) and patients with and without GVHD of the skin. Data are shown as median values with interquartile ranges and were all taken 12 weeks after allogeneic HSCT (n = 40 [cohort 2, Table 1]). *P < .05, **P < .01, ***P < .001. HC, healthy controls (black bars, n = 8).
Tissue-homing marker expression after allogeneic HSCT. (A) Expression of the gut-homing marker α4β7 by the ILCs of patients with high or low proportions of CD69+ ILCs, 12 weeks after allogeneic HSCT. (B) Expression of the gut-homing marker α4β7 in healthy controls (black bars) and patients with and without acute GVHD of the gut. (C) Proportion of CLA+ (left panels) and CCR10+ (right panels) ILC1 and NCR– ILC3 in healthy controls (black bars) and patients with and without GVHD of the skin. Data are shown as median values with interquartile ranges and were all taken 12 weeks after allogeneic HSCT (n = 40 [cohort 2, Table 1]). *P < .05, **P < .01, ***P < .001. HC, healthy controls (black bars, n = 8).
After allogeneic HSCT, reconstituting ILCs are of donor origin
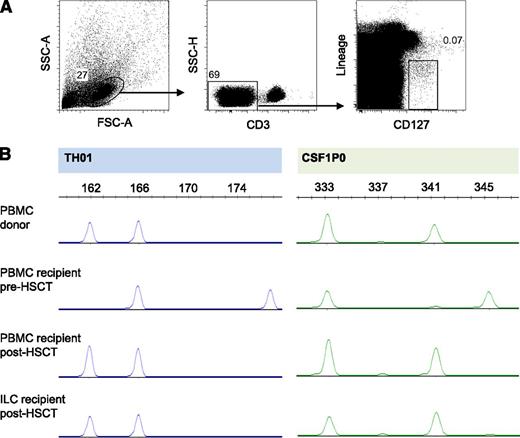
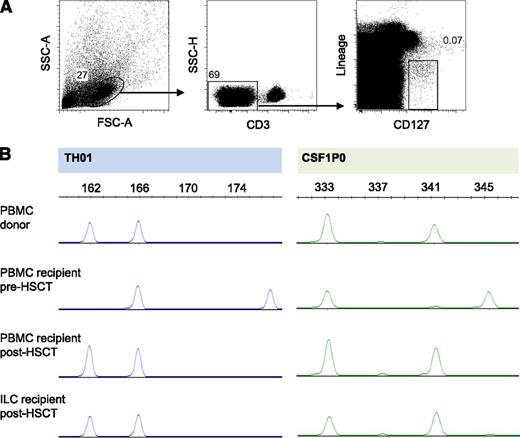
In a previously described murine model of allogeneic HSCT and acute GVHD, tissue-protective ILCs were of the recipient origin, suggesting that these ILCs were radiotherapy resistant.24 In our study, all patients included had obtained full-donor chimerism of PBMCs 12 weeks after allogeneic HSCT, with most patients showing full-donor chimerism as early as 4 weeks after HSCT. We analyzed genomic polymorphisms through profiling of STR DNA loci of sort-purified ILCs and demonstrated full-donor chimerism of peripheral blood ILCs in 5 of 6 allogeneic HSCT patients tested, as early as 7 weeks post-HSCT (Figure 6). One patient who had 90% donor chimerism of PBMCs also had 90% donor chimerism of ILCs. These data suggest that in humans, reconstituting ILCs after allogeneic HSCT are of donor origin.
After allogeneic HSCT, reconstituting ILCs are of donor origin. (A) Sorting strategy for ILCs of one HSCT recipient. (B) STR polymorphism profiles of sorted ILCs and of PBMCs of the same HSCT recipient before and after allogeneic HSCT, and of the donor for this patient. Demonstrated is one representative patient of 6 patients tested.
After allogeneic HSCT, reconstituting ILCs are of donor origin. (A) Sorting strategy for ILCs of one HSCT recipient. (B) STR polymorphism profiles of sorted ILCs and of PBMCs of the same HSCT recipient before and after allogeneic HSCT, and of the donor for this patient. Demonstrated is one representative patient of 6 patients tested.
Discussion
In the past years, ILCs have received increasing attention as a group of innate effector cells that, through the production of cytokines, mediate a variety of effects including the maintenance of tissue homeostasis and epithelial barrier integrity. Allogeneic HSCT is often complicated by GVHD that is characterized by allo-immune inflammation at the site of epithelial barriers such as the gut and skin and is elicited by damage to these tissues (eg, because of chemotherapy and radiotherapy). Given that in a mouse model for allogeneic HSCT–induced acute GVHD, radio-resistant ILCs protected tissues against damage inflicted by GVHD,24 we longitudinally analyzed ILC numbers, activation status, and expression of tissue-homing molecules in acute leukemia patients after induction chemotherapy and after allogeneic HSCT.
In a first set of experiments, we analyzed the effects of induction chemotherapy on the number and composition of ILCs. We observed that chemotherapy led to a significant loss of circulating ILCs, indicating that ILCs are not resistant to this regimen. Subsequent recovery of ILC1, ILC2, and NCR– ILC3 was slow. In contrast, circulating NCR+ ILC3 were present within weeks after induction chemotherapy, whereas such cells are not present in the circulation of healthy persons. A significant proportion of these NCR+ ILC3 expressed the gut-homing marker α4β7 and the skin-homing markers CLA and CCR10. Interestingly, pre-HSCT proportions of CD69+ NCR+ ILC3 that express homing receptors were significantly higher in patients who did not develop acute or chronic GVHD. The exact mechanism has yet to be elucidated, but it is possible that epithelial damage evoked by chemotherapy induces activation and proliferation of NCR+ ILC3, which then redistribute into the circulation. Thus the presence of activated CD69+ NCR+ ILC3 in the circulation may be a reflection of ongoing functional activities in the tissues of the patients. In the mouse, NCR+ ILC3 are known to be involved in tissue repair and prevention of bacterial translocation in the gut.35 It is likely that human NCR+ ILCs also mediate these functions in the gut, and together these activities may render the patients less sensitive to GVHD. We have demonstrated previously that human CD69+ ILCs derived from adult intestine produce IL-22,36 and perhaps IL-22 is an important mediator of protection against GVHD. This hypothesis is supported by the observation that IL-22–producing ILCs protected the gastrointestinal epithelium against GVHD-mediated tissue injury in an allogeneic HSCT mouse model.24
Patients with a lower incidence of GVHD after HSCT also had high proportions of circulating, activated CD69+ tissue-homing ILC2 and NCR– ILC3 after induction chemotherapy, before HSCT, suggesting that these activated ILCs may also be involved in protection against conditioning therapy–associated tissue damage, thereby impeding the development of GVHD. Indeed ILC2 have been shown to mediate tissue repair of damage inflicted by influenza virus infection of the lung through production of the EGF family member amphiregulin. However, whether amphiregulin-producing ILC2 and IL-22–producing NCR– ILC3 protect epithelial tissues from conditioning therapy–induced damage, lowering the probability of ensuing GVHD remains to be determined. If observations that high proportions of CD69+ ILCs before HSCT predict GVHD can be confirmed in larger cohorts of patients, this may be used as a biomarker to identify patients at risk of developing GVHD before allogeneic HSCT.
Second, we longitudinally analyzed ILC reconstitution after allogeneic HSCT. Appearing ILCs were of donor origin. Reconstitution of ILCs, in particular of ILC2, was slow in the patients under study, when compared with recovery of other innate effector cells such as neutrophils and monocytes. Similar slow recovery has been observed previously for T lymphocytes,37 which has been attributed to defects in the thymic environment. However, ILC2 can be detected in Foxn1ν/ν (nude) mice that do not have a functional thymus,17 making it unlikely that thymic damage accounts for the slow recovery of ILC2. It has also been suggested that lymphocyte recovery after allogeneic HSCT is the result of homeostatic proliferation of de novo–generated (from transplanted progenitor cells) and co-transplanted mature cells.38,39 Indeed the proliferation marker Ki-67 was highly expressed in all ILC subsets during reconstitution; however, Ki-67 expression did not correlate with the magnitude of ILC reconstitution. For example, ILC2 recovery was incomplete, despite high Ki-67 expression. In addition, Ki-67+ NCR+ ILC3 appeared after allogeneic HSCT, whereas these cells are virtually absent in the circulation of healthy individuals. These observations suggest that Ki-67 expression is not driven by homeostatic proliferation.
In healthy individuals, circulating ILCs do not express CD69, whereas this marker is expressed on ILCs in the tissues. The presence of CD69 on the circulating ILCs of patients recovering from HSCT may suggest that redistribution of ILCs from tissues contribute to ILC recovery. It remains to be determined to what extent ILC recovery, or the recovery of certain ILC subsets, depends on age. In our study, cohort 1 was age-matched with the control group, and recovery of circulating numbers of ILCs was incomplete 6 months after allogeneic HSCT (Figure 1C). Under homeostatic conditions, the majority of circulating ILCs consists of ILC2, and it was demonstrated recently that murine ILC2 are a long-lived population.40 Although the longevity of other ILC subsets remains to be determined, and the life span of human ILCs has not been assessed, it can be argued that when ILCs are a long-lived cell population, replacement of lost ILCs may be equally slow.
After allogeneic HSCT, reconstitution of ILCs was associated with a reduced incidence of GVHD. A rapid appearance of NCR+ ILC3 was observed in the peripheral blood of patients in whom acute or chronic GVHD did not develop. As discussed previously, NCR+ ILC3 mediate tissue protection and containment of intestinal commensals in mice, mechanisms that may be translatable to humans in the protection against GVHD. ILC3 may also mediate this effect by inhibition of T-cell activation because it was shown in the mouse that these cells express class II major histocompatibility complex and present antigen to T cells, leading to suppression of T-cell activation.41
It is possible that the lower numbers of recovering activated ILCs in patients in whom GVHD developed is caused by the GVHD itself or by immunosuppressive therapy to treat GVHD, but it should be noted that the dichotomy in NCR+ ILC3 reconstitution between patients with and without GVHD was apparent as early as 6 weeks after allogeneic HSCT, a time point when none of the patients under study had developed GVHD and all patients were receiving immunosuppressive treatment to prevent GVHD. In addition, we observed increased proportions of skin-homing ILC1 and NCR– ILC3 and gut-homing ILC2 in patients without acute GVHD of the skin and gut at the time of analysis, 12 weeks after allogeneic HSCT. Although this may suggest that ILC1, ILC2, and NCR– ILC3 also protect against the development of GVHD of gut and skin, similar to NCR+ ILC3, these data should be interpreted with caution. Early ILC recovery (6 weeks post-SCT) was measured in the smaller cohort only, and analyses of tissue-homing marker expression before 12 weeks after allogeneic HSCT are lacking, and GVHD itself or its treatment may affect the appearance of activated, tissue-homing ILC1, ILC2, and NCR– ILC3 (eg, by containing ILCs in inflamed tissues).
Of note, reconstituting ILCs after allogeneic HSCT were of donor origin. Before and after allogeneic HSCT, we observed an association of CD69+ ILCs with an absence of GVHD. It is tempting to speculate that patient ILCs protect epithelial tissues during the pre-HSCT conditioning chemotherapy and radiotherapy, thereby lowering the risk of later development of GVHD. In the weeks after allogeneic HSCT, these cells then disappear and are replaced by donor ILCs, which matches observations in a mouse model of GVHD.24 These donor ILCs, when appropriately activated in the tissues, may take over the role of recipient ILCs in protecting tissues against GVHD.
Although the present study represents the largest longitudinal characterization of ILC reconstitution performed to date, further studies in independent cohorts are needed to corroborate the link between ILCs and the emergence of GVHD. If confirmed, this may warrant supplying patients who undergo allogeneic HSCT with in vitro expanded NCR+ ILC3 and ILC2 to prove that these ILCs protect against GVHD. Techniques to culture and expand these ILCs have been developed in our laboratory12,42 and in that of others.43
The online version of this article contains a data supplement.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the patients and healthy volunteers for their participation in these studies, Berend Hooibrink and Toni van Capel for flow cytometry assistance, and Ludo Evers for STR analyses.
This study was supported by the Netherlands Organisation for Scientific Research (NWO ZonMW Clinical Fellowship, 40-00703-97-314 [M.D.H.]), an intramural grant of the Academic Medical Centre (J.M.M.), and the Academic Medical Center Foundation (anonymous donation).
Authorship
Contribution: J.M. Munneke designed and performed the research, analyzed the data, and wrote the paper; J.M. Mjösberg, J.H.B., B.B., and H.S. analyzed data, contributed critical input, and wrote the paper; A.T.B., K.J.-M., K.G.-L., C.H., and M.H.J.v.O. were responsible for patient management, provided critical input, and helped write the paper; and M.D.H. designed the research, managed the cohort, analyzed the data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Mette D. Hazenberg, Academic Medical Center, University of Amsterdam, Meibergdreef 9, Amsterdam, 1105 AZ, The Netherlands; e-mail: m.d.hazenberg@amc.nl.

![Figure 1. Loss and recovery of ILCs after AML induction chemotherapy and allogeneic HSCT. (A) Gating strategy for the identification of peripheral blood ILCs, here shown for a healthy individual. ILCs are Lin− (CD1a−CD11c−CD14−CD19−CD34−CD123−TCRαβ−TCRγδ− BDCA2−FcεR1−CD94−), CD3− lymphocytes that are CD127+. Groups 1, 2, and 3 ILCs are identified by the expression of CRTH2, CD117, and NKp44. Numbers in quadrants indicate the percent cells in each throughout. (B) Representative dot plot of a peripheral blood sample obtained from a patient with AML, 18 days after the first-induction chemotherapy cycle. (C) Loss and recovery dynamics of total ILCs (Lin−CD127+) during 2 AML induction chemotherapy cycles (left panel, n = 11 [cohort 1, Table 1]) and after reduced-intensity conditioning allogeneic HSCT (right panel, n = 6 [cohort 1, Table 1]). Samples were taken during neutropenia (at day 21 of each induction chemotherapy cycle), during the recovery phases of the first and second induction chemotherapy cycles, and at the days of resubmission for the second (“cycle 2”) and third (“cycle 3”; good-risk AML patients only) induction chemotherapy cycles. Patients with intermediate or poor-risk AML proceeded to allogeneic HSCT after the second induction chemotherapy cycle, and samples were collected from these patients at the day of admission to receive pre-allogeneic HSCT conditioning therapy (“allo-HSCT”). Arrows with “cycle 1,” “cycle 2,” or “cycle 3/allo-HSCT” (left panel) indicate the first day of induction chemotherapy cycles or the first day of conditioning therapy preceding allogeneic HSCT; peripheral blood neutrophil numbers had completely recovered at these time points. Dashed lines represent the 95% confidence interval (CI) as measured for healthy, age-matched controls (n = 10). (D) The recovery dynamics of total ILC (Lin−CD127+), ILC1, ILC2, NCR− ILC3, and NCR+ ILC3 after induction chemotherapy (white bars) and 12 weeks after allogeneic HSCT (gray bars, n = 40 [cohort 2, Table 1]). Data in (C) and (D) are depicted as median values with interquartile ranges. *P < .05, **P < .01, ***P < .001. HC, healthy controls (black bars, n = 8).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/124/5/10.1182_blood-2013-11-536888/2/m_812f1.jpeg?Expires=1765972966&Signature=ULTp5Ru3wb~HCuvDPAibn3IVxn86bHDEoU8SXPi8TqCs-o5BVEUfbNU70zMzaDWT7hzErPCtdZmNh1ORFncAb0NdaYeEXDw7TEeqUb0L4H18jW~zl5M5XiO3qqN7-IY3Ef3AEw7aslCj2bEeiMUeOfj8V0JMCI5ifUPgGQEZhofGKnoLt9jEkKdKJ5j86cfK1YEx-yHtfhBD0po0ng70HVWzFOS5dyxxzXPQdsyi8EMyC0iI08PF9Jxpg4oVkBDG67zt1fmRjy1VVjjOo2EBOf3m3uK-onWFT0IQXInRMN4RYeVoyrLw-vLYghlWCwIqJ206mvu-Cj9sC3egFDYFHA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 3. Appearance of α4β7+ CD69+ ILCs after induction chemotherapy is associated with a lower incidence of GVHD. (A) CD69 expression separated patients into 2 distinct groups: patients with a high or low proportion of CD69+ ILCs, in particular for ILC2, NCR− ILC3, and NCR+ ILC3. (B) Patients who did not develop acute GVHD had significantly higher proportions of CD69+ ILC before allogeneic HSCT. (C) Kaplan-Meier curves for patients who did or did not develop acute GVHD, stratified according to the expression of CD69 by ILC before HSCT. CD69hi denotes patients with a proportion of CD69+ ILC higher than median;CD69lo patients with CD69+ ILC proportions lower than median (see [A] for individual data). (D) Expression of the gut-homing molecule α4β7 by the ILCs of patients with high or low proportions of CD69+ before allogeneic HSCT. (E) Patients in whom acute GVHD of the gut did not develop had higher proportions of α4β7+ ILC2 and ILC3 before allogeneic HSCT. Squares in (A) denote healthy persons, triangles HSCT recipients (before allogeneic HSCT). Data (n = 40 [cohort 2, Table 1]) in (B,D-E) are shown as median values with interquartile ranges. All samples were taken before allogeneic HSCT. *P < .05, **P < .01, ***P < .001. HC, healthy controls (black bars, n = 8). ND, not determined.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/124/5/10.1182_blood-2013-11-536888/2/m_812f3.jpeg?Expires=1765972966&Signature=cNFB08M7UvJ4pCQCn~swBcqK1Qu2M1-t6JHRGndUW62w5zUwHd9wOZbAlLfRHBS0rK4CUN4lle06NMB7pKMqtZzivIofR1Lvol~xjtRfOAVnVxJDwVbwP9-mKxcmKs5XtlPsIUGN6elLY2Z-ltkW4rmQ7o-AMFGwzrv~4BjFlP5V2-t95ontGRwm8iJsTsZBhLB7KzjKruDJ1vtF5kVssjh5148i34TpXlWzTo7xWlTsIwEULzqOLqu9npH4G5TQ0nsG72poVd20o49KkW1FoWg3n5i0-pyKgg7Mm5YY8trm0~JGmBjd27SvdObxTr2U9gBhlpT5DV3aCvKHyB0GMQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 4. Elevated numbers of circulating NCR+ ILC3 after HSCT in patients without GVHD. (A) Mucositis was scored daily during HSCT conditioning therapy and thereafter in 24 patients (enrolled in cohort 2 after the year 2007; Table 1). Most patients in whom acute GVHD developed had grade II or grade III mucositis during conditioning therapy, whereas most patients in whom acute GVHD did not develop had grade I or grade II mucositis. (B) NCR+ ILC3 numbers 12 weeks after allogeneic HSCT for patients with or without acute GVHD or chronic GVHD (n = 40 [cohort 2, Table 1]). (C) The dichotomy between circulating NCR+ ILC3 numbers for patients in whom chronic GVHD would and would not develop was apparent as early as 6 weeks after allogeneic HSCT, a time point at which acute GVHD had developed in none of the patients and all patients were on maximal immunosuppressive therapy to prevent GVHD (n = 6 [cohort 1, Table 1]). Data are shown as median values with interquartile ranges. *P < .05, **P < .01, ***P < .001. HC, healthy controls (n = 8 [cohort 2] in panel A and n = 10 [cohort 1] in panel B).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/124/5/10.1182_blood-2013-11-536888/2/m_812f4.jpeg?Expires=1765972966&Signature=Q~kPNlSUSXCKghsg7f3tTHigPP05Lnhae5SeQ~KyX2E3wUKP3C9SCRFJaUO3tMk2GS2o8n3P6e-mfFoH0M~1VrCxmsxZZmyWOhZ5FgGwJyhVQ0DRdKowwvuuKXtetR9Bhi5WmzJN90h4xyHdeu6WmyhQ~Pc3vE9J9vDwqMYiaDkgqx484cww6ur9hq~R2t~3Xu0B-zSc2ITHcC~F6MdUxWXlRq5Y7egmP~1zL-4OakekLe3hqRPGA-XskNI5HKBu338YIwahHG7MjnYkP2bvqPWoIOVdoqWldPASJvpfKY-DJnpKLxZqnl6PezrZ4PukVq85yR8TY2PBOYbe4AUWRw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 5. Tissue-homing marker expression after allogeneic HSCT. (A) Expression of the gut-homing marker α4β7 by the ILCs of patients with high or low proportions of CD69+ ILCs, 12 weeks after allogeneic HSCT. (B) Expression of the gut-homing marker α4β7 in healthy controls (black bars) and patients with and without acute GVHD of the gut. (C) Proportion of CLA+ (left panels) and CCR10+ (right panels) ILC1 and NCR– ILC3 in healthy controls (black bars) and patients with and without GVHD of the skin. Data are shown as median values with interquartile ranges and were all taken 12 weeks after allogeneic HSCT (n = 40 [cohort 2, Table 1]). *P < .05, **P < .01, ***P < .001. HC, healthy controls (black bars, n = 8).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/124/5/10.1182_blood-2013-11-536888/2/m_812f5.jpeg?Expires=1765972966&Signature=Tn~j0WLzflPTfyMVYjPlzW5YzvsjElgVTEVcvlgsj-6vsT0KGB9vi6Ntqx3ukoL8V-y7ZYxXMiRvyWc0bF46V8U98idJj2k2qfIlPsJneLFdY3CcsShTwuvX~KoZzS44TUdJbI4GMxXeapfCkDwxJznsa~yIE6k~Fz5-NKIGkes6wieZDyD0Ms6WcUvVQdG8TGigHZHazXfF3s4pA3BVDIhgcogBm9ok1DogTu5t0e4GUQIprcHYC9bfCmZM5KjTSRBZd4z1L7w0ST8z9FFtEmDG6ngCY7X7JWF1uPxP56yA~zmCV-qH0m3SHkS0U~4Dwci0yjjvC-2M0YQ2Y2cw7w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 1. Loss and recovery of ILCs after AML induction chemotherapy and allogeneic HSCT. (A) Gating strategy for the identification of peripheral blood ILCs, here shown for a healthy individual. ILCs are Lin− (CD1a−CD11c−CD14−CD19−CD34−CD123−TCRαβ−TCRγδ− BDCA2−FcεR1−CD94−), CD3− lymphocytes that are CD127+. Groups 1, 2, and 3 ILCs are identified by the expression of CRTH2, CD117, and NKp44. Numbers in quadrants indicate the percent cells in each throughout. (B) Representative dot plot of a peripheral blood sample obtained from a patient with AML, 18 days after the first-induction chemotherapy cycle. (C) Loss and recovery dynamics of total ILCs (Lin−CD127+) during 2 AML induction chemotherapy cycles (left panel, n = 11 [cohort 1, Table 1]) and after reduced-intensity conditioning allogeneic HSCT (right panel, n = 6 [cohort 1, Table 1]). Samples were taken during neutropenia (at day 21 of each induction chemotherapy cycle), during the recovery phases of the first and second induction chemotherapy cycles, and at the days of resubmission for the second (“cycle 2”) and third (“cycle 3”; good-risk AML patients only) induction chemotherapy cycles. Patients with intermediate or poor-risk AML proceeded to allogeneic HSCT after the second induction chemotherapy cycle, and samples were collected from these patients at the day of admission to receive pre-allogeneic HSCT conditioning therapy (“allo-HSCT”). Arrows with “cycle 1,” “cycle 2,” or “cycle 3/allo-HSCT” (left panel) indicate the first day of induction chemotherapy cycles or the first day of conditioning therapy preceding allogeneic HSCT; peripheral blood neutrophil numbers had completely recovered at these time points. Dashed lines represent the 95% confidence interval (CI) as measured for healthy, age-matched controls (n = 10). (D) The recovery dynamics of total ILC (Lin−CD127+), ILC1, ILC2, NCR− ILC3, and NCR+ ILC3 after induction chemotherapy (white bars) and 12 weeks after allogeneic HSCT (gray bars, n = 40 [cohort 2, Table 1]). Data in (C) and (D) are depicted as median values with interquartile ranges. *P < .05, **P < .01, ***P < .001. HC, healthy controls (black bars, n = 8).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/124/5/10.1182_blood-2013-11-536888/2/m_812f1.jpeg?Expires=1765972967&Signature=KRLZRcXSfXshE7LE3XkCOOpr-mwOF9VPK2f0IVC6l3aZG4vbwQZxtEHbE752hGOAMJBtBHD6DcL1o6Bh-t~4YRwVxf6QHZGrkPt82MBQkVwD3n61gRHKShKBI~MAZqra-~T4svR7QQJNDOIoTDt6BIJattk0KnEB-hZN2SAkSWl6OYJdQ8gTXAHvggLAubmlETVoC~xJcDWAHT2MKxvGYce4dgYBU3kRaFpwc5WM~48FOlmFaoMC~bUoxl-FZDSFHLvLQvvZk5jg3NKikM2c6nTpzJT~G9XnYaQt1YFpninpbRH4ga0VVPMXyKCXcI0NYaaLdkYW1~jSld~IwnATKg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 3. Appearance of α4β7+ CD69+ ILCs after induction chemotherapy is associated with a lower incidence of GVHD. (A) CD69 expression separated patients into 2 distinct groups: patients with a high or low proportion of CD69+ ILCs, in particular for ILC2, NCR− ILC3, and NCR+ ILC3. (B) Patients who did not develop acute GVHD had significantly higher proportions of CD69+ ILC before allogeneic HSCT. (C) Kaplan-Meier curves for patients who did or did not develop acute GVHD, stratified according to the expression of CD69 by ILC before HSCT. CD69hi denotes patients with a proportion of CD69+ ILC higher than median;CD69lo patients with CD69+ ILC proportions lower than median (see [A] for individual data). (D) Expression of the gut-homing molecule α4β7 by the ILCs of patients with high or low proportions of CD69+ before allogeneic HSCT. (E) Patients in whom acute GVHD of the gut did not develop had higher proportions of α4β7+ ILC2 and ILC3 before allogeneic HSCT. Squares in (A) denote healthy persons, triangles HSCT recipients (before allogeneic HSCT). Data (n = 40 [cohort 2, Table 1]) in (B,D-E) are shown as median values with interquartile ranges. All samples were taken before allogeneic HSCT. *P < .05, **P < .01, ***P < .001. HC, healthy controls (black bars, n = 8). ND, not determined.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/124/5/10.1182_blood-2013-11-536888/2/m_812f3.jpeg?Expires=1765972967&Signature=C01FDr7HbXpUmyFETmLv4Bk40dD~8U8A1rrLiLjVVVgm4AHSAJ9fd45Owf-cpkA5iPy2zt3h57dMHnWi72rS91~OAkpZm~xgT-Qkn12uKN1lz2d35CUxX1r4cY-auMrhYfi3ffUtbgUTshqVljIA6JqDU12ow3FpWgEmEJpJrwT4SoZDbk0M9bxKjm49vGKPYr-zica6xmTFvBZUVGfFepsaMMxw2cnDRwhONTUa6QcSD556qrrb8mCF6I8QYu77g2uXaM-kYqkQ--VKMfzQ58OM1oU952Wueb9lPVHrvPH4Udm22UmlPcRco1cwUbyu6GczQ3gfS941A2zBDuSf1A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 4. Elevated numbers of circulating NCR+ ILC3 after HSCT in patients without GVHD. (A) Mucositis was scored daily during HSCT conditioning therapy and thereafter in 24 patients (enrolled in cohort 2 after the year 2007; Table 1). Most patients in whom acute GVHD developed had grade II or grade III mucositis during conditioning therapy, whereas most patients in whom acute GVHD did not develop had grade I or grade II mucositis. (B) NCR+ ILC3 numbers 12 weeks after allogeneic HSCT for patients with or without acute GVHD or chronic GVHD (n = 40 [cohort 2, Table 1]). (C) The dichotomy between circulating NCR+ ILC3 numbers for patients in whom chronic GVHD would and would not develop was apparent as early as 6 weeks after allogeneic HSCT, a time point at which acute GVHD had developed in none of the patients and all patients were on maximal immunosuppressive therapy to prevent GVHD (n = 6 [cohort 1, Table 1]). Data are shown as median values with interquartile ranges. *P < .05, **P < .01, ***P < .001. HC, healthy controls (n = 8 [cohort 2] in panel A and n = 10 [cohort 1] in panel B).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/124/5/10.1182_blood-2013-11-536888/2/m_812f4.jpeg?Expires=1765972967&Signature=1~40xXiLWwnnah4KhCKRD0XmP0PrqNTKBghfeovAXSXpr8nAe5lLmwEbgSuPKMOJjPow6nf7FY-f867VyENLBjAeWhP0EeFu9i0HoAkYQmVI-TkG09H3JKY1-CQEwb6B5SyTEVV25eWSBVXBkp3hFNHzn~PBXjYe4C0PXsggfrv7hOkaZII6xzPVjWkSyD3DLaeLPTqxj2OUouivzZ8IHv-CWm0g-ZfEZI-MdDr9jgNRyo7X7y4laGoPY4Z~JzbzKJZSJKMRw5~4~oTktEai1~z7lpxYFhsyE8SuzZ7waVN2AXoxvtXzAFKl8DSMZkW~0wOfrTGRhvOQOTc0yJ6Yqg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 5. Tissue-homing marker expression after allogeneic HSCT. (A) Expression of the gut-homing marker α4β7 by the ILCs of patients with high or low proportions of CD69+ ILCs, 12 weeks after allogeneic HSCT. (B) Expression of the gut-homing marker α4β7 in healthy controls (black bars) and patients with and without acute GVHD of the gut. (C) Proportion of CLA+ (left panels) and CCR10+ (right panels) ILC1 and NCR– ILC3 in healthy controls (black bars) and patients with and without GVHD of the skin. Data are shown as median values with interquartile ranges and were all taken 12 weeks after allogeneic HSCT (n = 40 [cohort 2, Table 1]). *P < .05, **P < .01, ***P < .001. HC, healthy controls (black bars, n = 8).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/124/5/10.1182_blood-2013-11-536888/2/m_812f5.jpeg?Expires=1765972967&Signature=pb0LoQMzUy-BsoquVisEH-wdFBNej0qm54ff-3jvFXi4mcKAKlDlXYzTwDnIFUc~PvHoULr5WSmpgMgKB2TEeF5fJTCN4uBYKzQGNVID621BPNtxscYkHuLZAM1hbCpxVZ1Jlrej03lQB0hrMTfaENq39cv8RB72JON8~VGzs~ss0U6i84QwcN2NYm9PYyaH-GUpgmHAJXjHruecWBhaYKMRU~1LlPQY0qfzNdN8WUvS7S3znHPGvfDcj0pSysRPzD8ryFb7THMST2-tVZfrXww4PCsIp07fF5012wwgAkBgdYwT4MvY-7FJPj8gcATQy-cEbJDSa6SBXRIDKDRXgA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)