Key Points
Haploidentical transplantation with regulatory and conventional T-cell adoptive immunotherapy prevents high-risk acute leukemia relapse.
The GVL effect is separated from GVHD even across major HLA barriers.
Abstract
Posttransplant relapse is still the major cause of treatment failure in high-risk acute leukemia. Attempts to manipulate alloreactive T cells to spare normal cells while killing leukemic cells have been unsuccessful. In HLA-haploidentical transplantation, we reported that donor-derived T regulatory cells (Tregs), coinfused with conventional T cells (Tcons), protected recipients against graft-versus-host disease (GVHD). The present phase 2 study investigated whether Treg-Tcon adoptive immunotherapy prevents posttransplant leukemia relapse. Forty-three adults with high-risk acute leukemia (acute myeloid leukemia 33; acute lymphoblastic leukemia 10) were conditioned with a total body irradiation–based regimen. Grafts included CD34+ cells (mean 9.7 × 106/kg), Tregs (mean 2.5 × 106/kg), and Tcons (mean 1.1 × 106/kg). No posttransplant immunosuppression was given. Ninety-five percent of patients achieved full-donor type engraftment and 15% developed ≥grade 2 acute GVHD. The probability of disease-free survival was 0.56 at a median follow-up of 46 months. The very low cumulative incidence of relapse (0.05) was significantly better than in historical controls. These results demonstrate the immunosuppressive potential of Tregs can be used to suppress GVHD without loss of the benefits of graft-versus-leukemia (GVL) activity. Humanized murine models provided insights into the mechanisms underlying separation of GVL from GVHD, suggesting the GVL effect is due to largely unopposed Tcon alloantigen recognition in bone marrow.
Introduction
In patients with acute leukemia (AL) at high risk of relapse because of unfavorable cytogenetics, molecular markers and disease status (complete remission [CR] ≥2), the most powerful postremission therapy is hematopoietic stem cell transplantation (HSCT) from a matched sibling (MSD) or unrelated donor (MUD).1,2 When patients do not have a MSD or MUD, unrelated cord blood (UCB)3 and haploidentical-related donor (haplo)4 HSCTs are emerging as alternatives.
In eradicating malignancy, ie, the so-called graft-versus-leukemia (GVL) effect, clinical observations5,6 and experimental models7-9 established that the allogeneic immune system played a crucial role. Donor T cells recognize host alloantigens on leukemic cells, although hematopoietic-specific and leukemia-specific responses may also occur.10 They also mediate graft-versus-host disease (GVHD), a major cause of morbidity and mortality after HSCT.
In T-cell–replete HSCT, pharmacological immunosuppression for GVHD prophylaxis and treatment is nonspecific and only partially successful. More importantly, it may compromise the T-cell–induced GVL effect. Indeed, relapse is still the major cause of treatment failure in high-risk AL patients.11-14
To date, attempts to manipulate alloreactive T cells to spare normal cells while killing leukemic cells have been largely unsuccessful. In the search for strategies to separate GVHD and the GVL effect and prevent disease recurrence, attention has focused on a thymic-derived CD4+CD25+ FoxP3+ regulatory T-cell subpopulation (Tregs) that physiologically helps maintain immunological self-tolerance and immune homeostasis.15,16 Evidence from murine models of bone marrow transplantation across major histocompatibility class I and II barriers showed that coinfusion of conventional T lymphocytes (Tcons) with Tregs, whether freshly isolated,17-19 ex vivo–expanded polyclonal,20 or recipient type,21 suppressed lethal GVHD without impairing Tcon activity against malignant diseases.22,23 In high-risk AL patients undergoing full-haplotype mismatched transplantation without any posttransplant immunosuppression, we demonstrated that adoptive immunotherapy with donor FoxP3+ Tregs (2 × 106/kg) and broad repertoire Tcons (1 × 106/kg) almost completely prevented acute and chronic GVHD and favored posttransplant immunological reconstitution.24
To address the issue of leukemia relapse, the present study investigated whether Treg-Tcon adoptive immunotherapy provided a powerful Tcon-mediated GVL effect in the absence of GVHD.
Patients and methods
Patient inclusion criteria
The protocol was approved by the Umbria Regional Hospital Ethical Committee and registered as 0108. Adults (18-65 years) with high-risk AL were eligible for haplo-HSCT with Treg-Tcon adoptive immunotherapy if they did not have an MSD or MUD. Before enrollment, all patients provided written informed consent in accordance with the Declaration of Helsinki.
Donors
Donors included healthy family members with 1 HLA haplotype identical to the patient’s who were able to donate hematopoietic stem cells after treatment with granulocyte colony-stimulating factor (G-CSF) and undergo leukapheresis sessions for collecting hematopoietic stem cells, Tregs, and Tcons. A natural killer (NK)-alloreactive donor was preferentially selected when available (Table 1).
Transplantation procedure
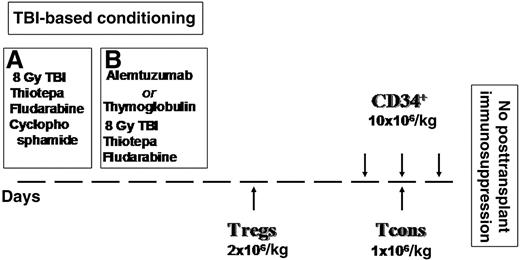
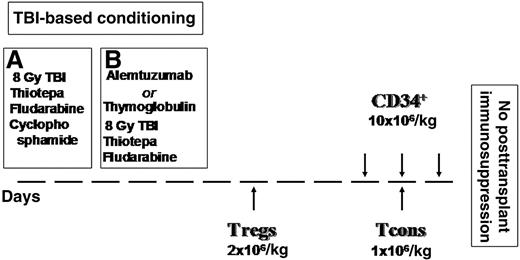
Figure 1 illustrates the transplant timeline schema. Briefly, the conditioning regimen included total body irradiation (TBI), thiotepa, and fludarabine. The first 25 patients also received cyclophosphamide. To reduce extrahematological toxicity and the risk of veno-occlusive disease, instead of cyclophosphamide the other 18 patients were given alemtuzumab (8 patients) or thymoglobulin (12 patients) when alemtuzumab was withdrawn from patients with hematological diseases. Anti-T antibodies were administered 21 days before transplant to prevent interference with Treg-Tcon adoptive immunotherapy. All patients received freshly isolated donor Tregs on day −4, followed by a megadose of purified CD34+ cells and Tcons on day 0. The 4-day interval between Treg and Tcon infusions was in accordance with animal data indicating that early Treg administration provided greatest protection against GVHD.18 No pharmacological posttransplant GVHD prophylaxis was given. Patients were managed according to our standard haplo-HSCT protocol.25
Conditioning regimen and inoculum. (A) Twenty-five patients (September 2008-October 2009): 8 Gy in a single fraction at a fast dose rate with lung shielding on day –10, thiotepa (4 mg/kg per day) on days –10 and −9, fludarabine (40 mg/m2 per day) from days –10 to –6, and cyclophosphamide (35 mg/kg per day) on days –7 and –6. (B) Eighteen patients (May 2010-December 2012): alemtuzumab lot #84039D 20 mg/m2 and thymoglobulin 3-7 mg/kg.
Conditioning regimen and inoculum. (A) Twenty-five patients (September 2008-October 2009): 8 Gy in a single fraction at a fast dose rate with lung shielding on day –10, thiotepa (4 mg/kg per day) on days –10 and −9, fludarabine (40 mg/m2 per day) from days –10 to –6, and cyclophosphamide (35 mg/kg per day) on days –7 and –6. (B) Eighteen patients (May 2010-December 2012): alemtuzumab lot #84039D 20 mg/m2 and thymoglobulin 3-7 mg/kg.
Graft processing
Tregs and Tcons were collected from donors before they underwent G-CSF treatment of CD34+ cell collection. Two total blood volumes from a single leukapheresis procedure were processed with COBE Spectra (Terumo BCT, Lakewood, CO). Tcons were separated from peripheral blood mononuclear cells by negative selection using CliniMACS CD19 reagent (Miltenyi Biotec) and cryopreserved.24 Naturally occurring CD4+CD25+ FoxP3+ Tregs were selected by means of a fully automated immunomagnetic procedure (Miltenyi Biotec GmbH, Bergisch Gladbach, Germany) by depleting the leukapheresis product of CD8+/CD19+ cells and then positively immunoselecting CD25+ cells.24 The initial leukapheresis products contained a median of 12.1 × 109 (range 5.4-18 × 109) nucleated cells. After magnetic cell separation, a median of 272 × 106 (range 142-412 × 106) Tregs was recovered.
Immediately after Treg and Tcon collection, donors were treated with G-CSF to mobilize peripheral blood progenitor cells. After collection, CD34+ cells were positively immunoselected using the CliniMACS device (Miltenyi Biotec).25 The target numbers of Tregs, Tcons, and CD34+ cells were achieved for all patients (see the following section). Phenotypes were determined using direct immunofluorescence with a panel of monoclonal antibodies as described previously.24 Overall, 3 days of apheresis were needed for each donor (1 for Treg and Tcon collection; 2 for CD34+ cells).
Suppression assays
Treg suppressive capacity was established as follows. Carboxyfluorescein diacetate succinimidyl ester (CFSE)-labeled CD4+/CD25− T lymphocytes were cultured in 96-well plates at 2 × 104 cells/well with phytohemagglutinin (PHA; 4 μg/mL; Biochrom) in the presence of varying numbers of CD4+/CD25+ (Tregs). For a CFSE-based measurement of proliferation, the suppressive capacity of Tregs toward responder cells in coculture (a Tcon:Treg ratio of 1:1 or 1:2) was expressed as the relative inhibition of the percentage of CFSElow cells as follows: 100 × (1 − %CFSElowCD4+CD25− T cells in coculture/%CFSElowCD4+CD25− T cells alone).
Chimerism analysis and immunological studies
Chimerism was assessed on DNA extracted from peripheral blood samples by multiplex fluorescent short-tandem repeat analysis. Peripheral blood was collected weekly for the first 2 months and then monthly for cytometric lymphocyte subset immunophenotyping. Alloreactive NK cell subsets were phenotyped in donors and monthly in recipients after transplant. Function was analyzed in cytotoxicity assays against KIR ligand mismatched recipient target cells to determine the frequency of alloreactive NK clones.24
End points and definitions
The primary end point was to assess the impact of Treg/Tcon immunotherapy in haploidentical transplantation on the cumulative incidence of posttransplant leukemia relapse. Disease status was assessed in bone marrow in all transplant recipients on days 30, 60, 90, 120, 180, 240, and 360, and then annually. Relapse was defined as disease recurrence according to the following: marrow morphology, flow cytometry, cytogenetics including fluorescence in situ hybridization and polymerase chain reaction for disease markers. The secondary end points were full donor type engraftment; probability of disease-free survival (DFS) based on patients who were alive without evidence of disease. Incidence of grades 2-4 and 3-4 acute GVHD on day +100; chronic GVHD at 1 year; cumulative incidence of nonrelapse mortality (NRM) (ie, death by any cause in the absence of disease relapse); GVHD mortality included patients with a history of GVHD who died from infections or organ failure during immunosuppressive therapy.
Historical controls
We compared outcomes of the present Treg-Tcon transplants with 114 high-risk CR1, ≥CR2 AL patients (50 acute lymphoblastic leukemia [ALL]; 64 acute myeloid leukemia [AML]; median age 37 years) (Table 1) from previous haploidentical-HSCT trials25-27 All had received a similar conditioning regimen with TBI, thiotepa, cyclophosphamide/fludarabine, and thymoglobulin that, unlike the present series, was administered during conditioning and not 21 days earlier. The inoculum included a megadose (mean CD34+ cells 10 × 106/kg ± 2.1) of hematopoietic progenitor cells. The contaminating T lymphocytes ranged in number from 1 × 104 to 1 × 105/kg body weight.
Statistical analyses
Cumulative incidence estimates (evaluated by the Gray test) were used for relapse and nonleukemic mortality because they are competing risks. The Kaplan-Meier method evaluated event-free survival. The log-rank test assessed impact of disease (AML vs ALL), disease status, gender, age, pretransplant donor-recipient pair cytomegalovirus (CMV), and donor-recipient NK cells on event-free survival. Multivariate analysis (Cox regression model) also investigated the impact of these factors on nonleukemic mortality, relapse, and event-free survival. Two-sided P values <.05 were considered significant.
Mouse models
Colonies of nonobese diabetic-scidIl2rgtm (NSG) mice were bred at the Perugia University Animal House. All experiments were performed in accordance with the National Ethics Approval Document for animal experimentation.
NSG mice were irradiated with 3.5 Gy and infused IV with human primary AML cells (7 × 106/mouse), human common Ph1 ALL cell line (SUP-B15, 2 × 106/mouse), or human Burkitt-like cell line (Namalwa, 2 × 106/mouse). After primary AML leukemia engraftment, mice were coinfused with human Tcons (3 × 106/mouse) and Tregs (3 × 106/mouse). In mice treated with lymphoblastic cell lines, Tregs and T cons were infused with leukemic cells. Controls were either untreated or infused with human Tcons (3 × 106/mouse) or Tregs (3 × 106/mouse). Tregs and Tcons came from the same donor and were HLA-mismatched with the primary leukemia and leukemia cell lines. At different time points, mice were evaluated for GVHD (by observation score and histopathology), leukemia infiltration, and T-cell homing (by flow cytometry with a combination of anti-human monoclonal antibodies: CD45, CD25, FoxP3, CD34, CD13 [eBioscience, CA]; CD3, CD56, CD8, CD4, CD14 [Miltenyi, Koln, Germany]; CD33, CD19, CD20, CD10 [Beckman Coulter, Marseilles, France]; and CD117, CD7, kappa, lambda, and immunoglobulin M (Invitrogen, CA). Each group comprised 5 mice. All experiments were repeated in duplicate. Results of duplicated experiments were combined.
Human CD3 T cells were purified from murine bone marrow by anti-human CD3-conjugated microbeads (Miltenyi) and assessed in a chromium release cytotoxicity assay with a 5:1 ratio as an effector against the targets (leukemia and autologous to leukemia PHA blasts).
Results
Patients and graft characteristics
Forty-three consecutive AL patients (median age 40 years; range 18-65) were treated between September 2008 and December 2012. Thirty-three had AML (18 CR1, 15 ≥CR2), 10 had ALL (7 CR1; 3 in ≥CR2). Twenty-four of these patients have already been reported in a previous article.24 All patients who were transplanted in CR1 were at high risk of relapse (8 FLT-3/ITD, 8 complex karyotypes, 4 with t(9:22), 2 primary induction failures, 1 secondary AML, 1 CR after second-line induction, and 1 with central nervous system and skin involvement at diagnosis).
All patients received a full haplotype mismatched graft that included CD34+ cells (mean 9.7 × 106/kg ± 3.1; mean contaminating T cells: 0.8 × 104/kg ±0.4), Tregs (mean 2.5 × 106/kg ±1), and Tcons (mean 1.1 × 106/kg ± 0.6). The Treg phenotype was Foxp3+ 81.01% ± 16.47, Helios/FoxP3+ 54% ± 8.4, and CD127+ 11.72% ± 7.653. CD45RO+ cells were 90% and CD45RA+ cells 10%. Treg suppressive capacity in vitro was 67% ± 22 (± standard deviation) (ratio Tcons:Tregs 1:2). Infused Tcons were 90.72% ± 9.6 CD3+, 57.77% ± 8.85 CD4+, and 31.21% ± 8.59 CD8+.
Table 1 shows the demographic characteristics and disease status of the present series of patients vs the historical control group. Median age, the proportion of AML vs ALL cases ,and remission status were similar in the 2 cohorts. All patients in both cohorts who underwent transplantation in CR1 had high-risk features. Median time from diagnosis to transplantation was 6 months (range, 3-48 months in the present cohort) vs 5 months (range, 2-60 months in the historical controls); P = .4. For patients who underwent transplantation in CR2, duration of remission was 6.5 months (range, 1-60 months present series) vs 7 months (range, 2-55 months historical controls); P = .6).
Engraftment and posttransplant immune recovery
Forty-one of 43 patients achieved primary, sustained full-donor type engraftment. Neutrophils reached 1 × 109/L (median 16 days; range 10-39). Platelets reached 20 × 109/L and 50 × 109/L (medians 13 and 15 days, respectively; ranges 8-48 and 13-60). The incidence of sustained engraftment was similar for Treg/Tcon vs historical control patients (95% vs 90%; P = .8). Likewise, time to neutrophil and platelet recovery was not different between Treg/Tcon and historical controls (P = .11).
There was a rapid, sustained increase in peripheral blood T-cell subpopulation recovery: CD4+ and CD8+ cells reached 50/µL at a median of, respectively, 30 days (range 16-65) and 25 (range, 12-90); 100/µL at a median of 40 (range 25-150) and 45 days (range 18-100); and 200/µL at a median of 55 (range 45-160) and 60 days (range 50-140). Compared with standard haploidentical transplantation, specific CD4+ and CD8+ for opportunistic pathogens such as Aspergillus fumigatus, Candida albicans, CMV, adenovirus, herpes simplex virus, and varicella zoster virus toxoplasma emerged significantly earlier (at each time point P < .0001).24
NK cells reached 200/µL at a median of 25 days (range 19-35) and 400/µL at a median of 45 days (range 22-70). Posttransplant alloreactive NK clones against KIR ligand–mismatched targets had a higher frequency than in historical controls (15 ± 6 vs 7 ± 5 at 1 month posttransplant).
GVHD and NRM
Only 6/41 patients (15%) developed ≥grade 2 acute GVHD. In 2, GVHD responded rapidly to a short course of immunosuppression, and only 1 of these patients developed chronic GVHD. Even though 1.1 × 106/kg ± 0.6 Tcons were infused, the incidence of ≥grade 2 acute GVHD was similar (P = .2) to the 11% in historical controls. They had received extensively T-cell–depleted grafts and antithymocyte globulin, which exerted additional in vivo T-cell depletion. Three patients (7.5%) died of GVHD (1 grade 2 acute GVHD, 2 grade 3-4 acute GVHD) compared with 10 (9%) in the historic cohort.
Overall, in the present series, the cumulative incidence of NRM was 0.40, which fell to 0.21 in 18 patients who received anti-T antibodies during conditioning. Causes of nonrelapse death were: veno-occlusive disease in 3 patients who were heavily pretreated until a few days before transplantation, GVHD (3), adenoviral infection (2), bacterial sepsis (2), HHV6 infection (2), multiorgan failure (1), and systemic toxoplasmosis (1). Lung (3) and central nervous system (1) aspergillosis occurred in patients with invasive fungal disease before transplantation.
Relapse and DFS
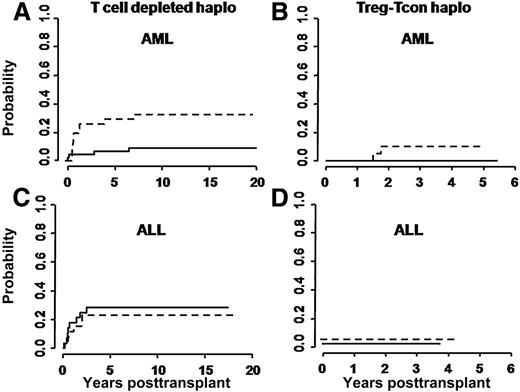
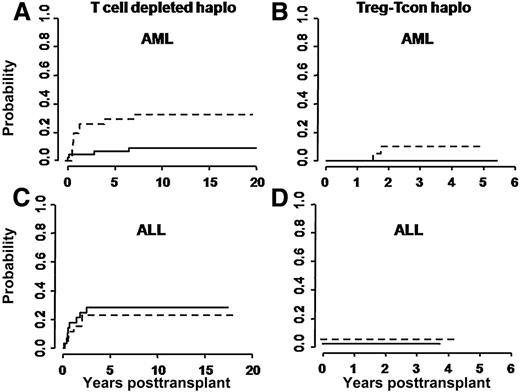
At a median follow-up of 46 months (range 18-65 months), only 2/41 evaluable patients have relapsed. Both had NPM+FLT3+ AML in molecular relapse at time of HSCT and had received a transplant from non–NK-alloreactive donors. Risk of relapse was not related to modification of the treatment plan over time. In 114 historical controls, relapse occurred in 11/50 patients with ALL, in 10/32 patients with AML transplanted from non–NK-alloreactive donors, and in 4/32 AML patients who were transplanted from NK-alloreactive donors. Figure 2 reports relapse rates in the Treg-Tcon–treated patients and historical controls who underwent haplo-HSCT from NK-alloreactive and non–NK-alloreactive donors.
Cumulative incidence of posttransplant leukemia relapses. (A,C) Relapses in historical controls, and (B,D) in the present cohort of Treg-Tcon patients. Relapse rates were evaluated separately for transplants from NK alloreactive (solid line) vs non–NK-alloreactive (dotted line) donors. In historical AML patients, the cumulative incidence of relapse was significantly lower for those who were transplanted from NK-alloreactive donors (0.32 vs 0.03; P = .03), whereas no difference was observed in historical ALL patients (0.31 and 0.29, respectively). In Treg-Tcon haplo transplants leukemia relapse was markedly reduced in all patients independently of NK alloreactivity (B,D).
Cumulative incidence of posttransplant leukemia relapses. (A,C) Relapses in historical controls, and (B,D) in the present cohort of Treg-Tcon patients. Relapse rates were evaluated separately for transplants from NK alloreactive (solid line) vs non–NK-alloreactive (dotted line) donors. In historical AML patients, the cumulative incidence of relapse was significantly lower for those who were transplanted from NK-alloreactive donors (0.32 vs 0.03; P = .03), whereas no difference was observed in historical ALL patients (0.31 and 0.29, respectively). In Treg-Tcon haplo transplants leukemia relapse was markedly reduced in all patients independently of NK alloreactivity (B,D).
In the Treg-Tcon cohort, the cumulative incidence of relapse was significantly lower than in historical controls (0.05 vs 0.21; P = .03). Multivariate analysis identified Treg-Tcon adoptive immunotherapy as the only predictive factor associated with a reduced risk of relapse (relative risk 0.06; 95% confidence interval, 0.02-0.35; P = .02).
Twenty-three of 41 patients are alive and well with DFS probability of 0.56 at 18 months’ minimum follow-up. DFS in the control cohort was 0.39 (P = .07), indicating a trend toward better survival under the Treg/Tcon protocol.
Murine studies
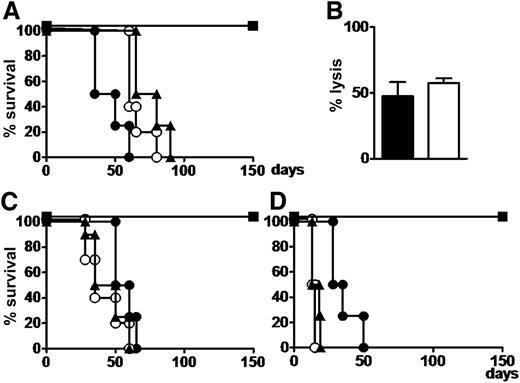
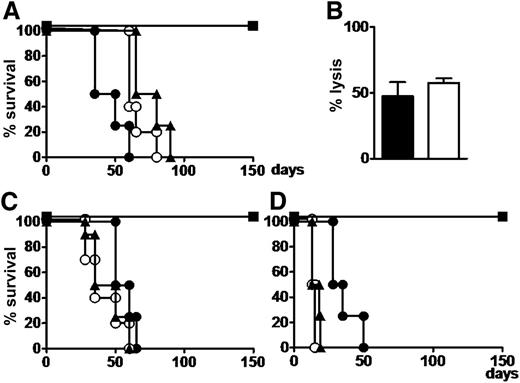
All mice that received myeloid or lymphoblastic leukemias, Tregs, and Tcons were rescued from leukemia and survived without GVHD. Cytofluorimetric analysis of all organs and tissues showed disease eradication. All mice that received leukemic cells with or without Tregs died of leukemia within 60 to 75 days. Those that received leukemia cells plus Tcons developed severe GVHD and died within 60 days (P < .002 mice with Treg/Tcon infusions vs all others).
Human T cells from bone marrow in mice that were rescued from leukemia by Treg-Tcon adoptive immunotherapy exhibited similar cytotoxicity against both leukemic and PHA blast cells that were autologous to leukemia (Figure 3). This experiment was repeated twice.
Coinfusion of human conventional Tcons and Tregs exerts a GVL effect without GVHD in mice with human leukemia. (A) NSG mice were given 3.5 Gy TBI and then infused with 7 × 106 primary human AML cells. Thirty days after leukemia engraftment, mice were treated with 3 × 106 Tcons and/or 3 × 106 Tregs. Untreated (○) or Treg-infused (▲) mice died of leukemia; Tcon-treated mice (●) died of GVHD; mice coinfused with 3 × 106 Tcons and 3 × 106 Tregs (▪) survived without GVHD. (B) Human T cells harvested from bone marrow of mice treated with the Tcon plus Treg combination exerted allogeneic lysis against leukemia (black bar) and PHA blasts (white bar) autologous to leukemia to an effector to target ratio = 5:1 in a chromium-releasing cytotoxicity assay. (C) Mice infused with 2 × 106 Ph+ cell line cells (SUP-B15) and treated with 3 × 106 Tcons and 3 × 106 Tregs (▪) survived leukemia without GVHD. Untreated mice and those infused with 3 × 106 Tregs (▲) died of leukemia within 60 days; mice infused with 3 × 106 Tcons (●) died of GVHD. (D) Similar outcomes were obtained with Burkitt’s cell line (Namalwa). All experiments were conducted in duplicate in groups of 5 mice.
Coinfusion of human conventional Tcons and Tregs exerts a GVL effect without GVHD in mice with human leukemia. (A) NSG mice were given 3.5 Gy TBI and then infused with 7 × 106 primary human AML cells. Thirty days after leukemia engraftment, mice were treated with 3 × 106 Tcons and/or 3 × 106 Tregs. Untreated (○) or Treg-infused (▲) mice died of leukemia; Tcon-treated mice (●) died of GVHD; mice coinfused with 3 × 106 Tcons and 3 × 106 Tregs (▪) survived without GVHD. (B) Human T cells harvested from bone marrow of mice treated with the Tcon plus Treg combination exerted allogeneic lysis against leukemia (black bar) and PHA blasts (white bar) autologous to leukemia to an effector to target ratio = 5:1 in a chromium-releasing cytotoxicity assay. (C) Mice infused with 2 × 106 Ph+ cell line cells (SUP-B15) and treated with 3 × 106 Tcons and 3 × 106 Tregs (▪) survived leukemia without GVHD. Untreated mice and those infused with 3 × 106 Tregs (▲) died of leukemia within 60 days; mice infused with 3 × 106 Tcons (●) died of GVHD. (D) Similar outcomes were obtained with Burkitt’s cell line (Namalwa). All experiments were conducted in duplicate in groups of 5 mice.
Discussion
The present study is the first to analyze the impact of Treg-Tcon adoptive immunotherapy on AL disease eradication. Infusion of freshly isolated FoxP3+ Tregs in this large series of high-risk patients undergoing full-HLA haplotype mismatched transplantation controlled the alloreactivity of up to 1 × 106/kg T lymphocytes, which is about 2 log more than the threshold dose for GVHD. Indeed, nearly 90% of patients were protected against GVHD, thus confirming previous results.24 Brunstein et al, who employed ex vivo expanded polyclonal Tregs as supplemental GVHD prophylaxis in double-unit UCB transplantation, also reported a significantly reduced incidence of grade 2 to 4 GVHD.28
Present results and those of Brunstein et al suggest that adoptive immunotherapy with Tregs does not compromise general immunity or blunt responses to infectious agents. Immunological reconstitution in our transplant recipients was stronger and faster than in the historical controls. Prevention of CMV disease improved markedly, with no CMV-related deaths, which had been one of the major causes of mortality in the historical control group.
Overall, NRM is still unsatisfactory in the present series but it is interesting to note it dropped sharply in patients who received anti-T antibodies in the conditioning regimen. Better outcomes may have been linked to cyclophosphamide suspension because less extramedullary toxicity was associated with lack of severe veno-occlusive disease and multiorgan failure. In the future, to reduce the risk of posttransplant infection–related morbidity and mortality, it might be worth increasing the number of Tcons in the graft by means of CD3+CD45RO+ cells,29 thus approaching what is termed a “designed” graft for haploidentical transplantation.30
One major concern about the use of Tregs in HSCT is their potential to suppress antineoplastic immune responses, as suggested by some studies in solid tumors31,32 and hematological malignancies.33,34 For example, Tregs accumulated in leukemic sites in mice, preventing adoptively transferred anti-AML T cells from proliferation and cytolysis.35 Conversely, interleukin-2 diphtheria toxin, which depleted Tregs expressing CD25, increased the number of cytotoxic T lymphocytes at tumor sites and resulted in tumor regression.36
On the other hand, in mismatched transplant mouse models, adoptive immunotherapy with Tregs and Tcons protected mice from GVHD without impairing Tcon control of neoplastic cell line expansion, such as A20 leukemic cells of BALB/c origin,22,23 BCL1 lymphoma,22 and P815 mastocytoma.23 The present study shows for the first time in humanized mouse models that human Treg-Tcon immunotherapy eradicated human primary myeloid leukemia and lymphoblastic leukemia cell lines without triggering GVHD.
In the present series of AL patients, infusion of Tcons under the Treg protective umbrella, in the absence of posttransplant pharmacological immunosuppression, guaranteed almost total control of AML and ALL relapse. The cumulative incidence of posttransplant leukemia relapse was 0.05 at a medium follow-up of 46 months, which is extremely low considering these patients were at high risk of relapse according to cytogenetics, molecular markers, and disease stage at transplant. In our historical controls, relapse rates were >30% in high-risk ALL patients and in AML patients who were not transplanted from NK alloreactive donors. Similarly, relapse rates ranged from 28% to 40% in recent clinical trials of T-cell–replete HSCT, independently of whether the donor was a MSD, MUD, UCB, or haplo.11-14 In the present series, the T-cell–dependent GVL effect was so powerful that it masked the anti-AML activity exerted by posttransplant generation of alloreactive donor-versus-recipient NK cell repertoires.37-39
The mechanisms underlying Treg suppression of GVHD with no loss of GVL activity are still obscure. In animal models, Edinger et al observed that Tregs inhibited early expansion of alloreactive donor T cells and their capacity to induce GVHD but did not inhibit cotransplanted Tcon activation and cytotoxicity against leukemia and lymphoma cells in vitro and in vivo. Thus GVL activity appears to rely mainly on Tcon activation rather than expansion and consequently requires transplantation of sufficient Tcons.22
In our murine models, human T cells that were harvested from bone marrow killed human leukemia cells and autologous PHA blasts in vitro, confirming their cytotoxicity activity was preserved and suggesting the T-cell–dependent GVL effect is mainly due to alloantigen recognition.
The GVL effect in the absence of GVHD could also be related to Treg migratory properties that are linked to expression of homing molecules for diverse sites. Tracking Treg in vivo dynamics in animal models of incompatible transplantation showed that upon infusion of polyclonal Tregs, alloantigen-specific Treg were activated and expanded in lymph nodes and then migrated to peripheral tissues (skin, gut, liver, lung).18 Thus alloantigen-specific Tregs controlled Tcon alloreactivity not only in lymph nodes but in nonlymphoid tissues, which are GVHD targets.
In the clinical transplant setting, one might hypothesize the GVL effect is due to largely unopposed Tcon alloreactivity in bone marrow. In fact, few infused donor Tregs are likely to migrate there because they are almost 100% RO+ with very low CXCR4 expression. In humans, Booth et al showed CD45RO+ Tregs home to skin, whereas CD45RA+ Tregs expressing CXCR4 preferentially localize in bone marrow.40 There, according to Fujisaki et al, they provide immune privilege for the hematopoietic stem cell niche, thus protecting hematopoietic stem/progenitor cells against Tcon alloreactivity. Interestingly, in this mouse transplant model, Treg depletion in bone marrow was associated with Tcon-induced loss of hematopoietic stem/progenitor cells.41 Similarly, in our setting, failure of donor Tregs to home to the bone marrow might have exposed leukemic stem cells to donor T-cell alloreactivity.
Whatever the mechanisms, with Treg adoptive immunotherapy, there is no need for posttransplant pharmacological prophylaxis against GVHD that, as we know, could impair the GVL effect. Thus, without the risk of Tcon activity being inhibited, 1 × 106/kg T-effector cells are sufficient to eradicate minimal residual disease. Consequently, the 30% to 35% incidence of relapse, which is generally reported by conventional T-cell–replete HSCT in high-risk AL patients, is practically eliminated.
Present results also demonstrate that adoptive immunotherapy with Tregs does not require ex vivo Treg expansion systems,42,43 which are cumbersome, expensive, and require good manufacturing practices facilities.
In conclusion, we are confident the innovative use of Tregs and Tcons is a major advance over conventional HSCT that, as leukemia immunotherapy, is associated with severe side effects and is only partially efficacious. In the future, the present strategy might be applied to exploit the GVL effect of Tcons that recognize minor histocompatibility antigens in matched-donor HSCT.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Geraldine Anne Boyd for editorial assistance.
This work was supported by a Translational Research Grant from the Leukemia and Lymphoma Society and by funding from Italian Association for Cancer Research, Associazione Umbra Leucemie e Linfomi, Italian Ministry of Further Education (Progetto di Rilevante Interesse Nazionale no. 2010NECHBX_005), Italian Ministry of Health (European Research Area Net Translational Cancer Research, no. PER-2011-2353844), and the European Union 7th Framework Program (as part of the “Nano II” project, no. 229289). L.R. is a Leukemia and Lymphoma Society Scholar in Clinical Research.
Authorship
Contribution: M.F.M., M.D.I., L.R., and A.V. designed the study, oversaw the results, and drafted the paper; Y.R., F.A., and B.F. contributed to the design and interpretation of the study; A.C., A.T., A.P., M.S.M., and L.A. provided clinical data; and L.R., F.F., E.U., B.D.P., T.Z., R.I.O., D.C., and R.T. performed the in vitro studies and interpreted the results.
Conflict of interest disclosure: the authors declare no competing financial interests.
Correspondence: Mauro Di Ianni, Hematology Section, Department of Life, Health and Environmental Sciences, University of L’Aquila, Italy; e-mail: mauro.diianni@cc.univaq.it.
References
Author notes
M.D.I. and L.R. contributed equally to this study.