Key Points
BMSCs and MM cells mutually communicate through exosomes, which carry selective cytokines.
BMSC-derived exosomes favor MM cell proliferation, migration, and survival and induce drug resistance to bortezomib.
Abstract
The interplay between bone marrow stromal cells (BMSCs) and multiple myeloma (MM) cells performs a crucial role in MM pathogenesis by secreting growth factors, cytokines, and extracellular vesicles. Exosomes are membranous vesicles 40 to 100 nm in diameter constitutively released by almost all cell types, and they mediate local cell-to-cell communication by transferring mRNAs, miRNAs, and proteins. Although BMSC-induced growth and drug resistance of MM cells has been studied, the role of BMSC-derived exosomes in this action remains unclear. Here we investigate the effect of BMSC-derived exosomes on the viability, proliferation, survival, migration, and drug resistance of MM cells, using the murine 5T33MM model and human MM samples. BMSCs and MM cells could mutually exchange exosomes carrying certain cytokines. Both naive and 5T33 BMSC-derived exosomes increased MM cell growth and induced drug resistance to bortezomib. BMSC-derived exosomes also influenced the activation of several survival relevant pathways, including c-Jun N-terminal kinase, p38, p53, and Akt. Exosomes obtained from normal donor and MM patient BMSCs also induced survival and drug resistance of human MM cells. Taken together, our results demonstrate the involvement of exosome-mediated communication in BMSC-induced proliferation, migration, survival, and drug resistance of MM cells.
Introduction
Multiple myeloma (MM) is a deadly hematological malignancy characterized by the uncontrolled growth and accumulation of monoclonal plasma cells in the bone marrow (BM), the presence of a monoclonal immunoglobulin fraction in the serum or urine,1,2 renal failure, and osteolytic bone lesions.3 MM cells depend on the BM microenvironment for their growth and survival through interaction with the BM stromal cells (BMSCs). BMSCs consist mainly of fibroblasts and can secrete different kinds of cytokines, chemokines, growth factors, and small molecular mediators.4 These functional components trigger MM growth, survival, and progression through several signaling pathways, such as mitogen-activated protein kinase kinase/mitogen-activated protein kinase, focal adhesion kinase, phosphatidylinositol 3-kinase/Akt, MEK/extracellular signal-regulated kinase, and signal transducer and activator of transcription 3,5 which will ultimately lead to angiogenesis, bone disease, and drug resistance.
Exosomes are small (40-100 nm) membrane vesicles secreted by various cell types, including dendritic cells, B cells, T cells, mast cells, epithelial cells, and tumor cells,6 through the fusion of multivesicular bodies with the plasma membrane.7 Exosomes mediate local cell-to-cell communication by transferring mRNAs, miRNAs, and proteins. Because of their ability to transfer functional components, exosomes play multiple roles by stimulating target cells, transferring membrane receptors, delivering proteins, and inducing epigenetic changes in recipient cells.8 In addition to cell communication, they are also associated with immune response,9,10 antigen presentation,11 tumor survival,12 cell migration,13-15 tumor invasion,16 cell differentiation,17 and angiogenesis.13,15 Several signaling pathways such as Notch,15 phosphatidylinositol 3-kinase/Akt,7 Wnt,18 transforming growth factor (TGF)-β/Smad,17,19 Fas,16 and extracellular signal-regulated kinase 1/220 have been reported to be involved in exosome-induced physiological changes in recipient cells.
Although the promotion of MM growth and survival induced by BMSCs has been studied,21,22 the role of BMSC-derived exosomes in these actions remains unclear. In the present study, we investigated the effect of BMSC-derived exosomes on the viability, proliferation, survival, migration, and drug resistance of MM cells, using the murine 5T33MM model and human samples. The 5T33MM model presents similar clinical and biological characteristics of human MM, including the selective localization of the malignant cell in the BM, the presence of serum M-component, and increased angiogenesis.4,23 This similarity makes the 5T33MM model a suitable model to examine BM interactions.
Materials and methods
Mice, 5T33MM model, cell culture, reagents, and antibodies
All the details of mice, 5T33MM model, cell culture, reagents, and antibodies used in this study are presented in the supplemental Materials and methods, available on the Blood website.
Exosome isolation and NanoSight tracking analysis
Conditioned medium collected from 48-hour BMSC or MM cultures without serum were concentrated using Centriprep Centrifugal Filter 3K Devices (Millipore Corporation) and filtered using 0.22-μm pore filters, followed by incubation with ExoQuick-TC exosome precipitation solution (System Biosciences) at 4°C overnight. Exosomes were then harvested by centrifugation at 1500 g for 30 minutes and suspended in phosphate-buffered saline or serum-free medium. Sixteen microliters of cell lysis buffer were added into 4 μL exosome suspension, and the concentration of exosomal proteins was quantified using the Pierce BCA Protein Assay Kit (Thermo Scientific), according to the manufacturer’s instruction. Exosomes isolated as described earlier were characterized for particle size distribution and quantified by NanoSight LM10 (Nanosight). The data were processed using Nanoparticle Tracking Analysis (NTA) 2.2 analytical software.
Transmission electron microscopy
Exosomes were fixed in 2% paraformaldehyde and absorbed for 20 minutes to a Formvar-carbon coated grid. After adsorption, the samples were washed with PBS and transferred to a drop of 1% glutaraldehyde for 5 minutes. The grids were washed 8 times with Milli-Q water and negatively stained with 2% uranyl acetate for 5 minutes. Grids were dried and visualized on a TECNAI 10 transmission electron microscope (Philips) at 80 kV. The images were captured using iTEM software (Olympus Soft Imaging Solutions).
Western blot analysis, in vitro migration assay, cell viability, BrdU cell proliferation assay, and cell apoptosis assay
The details of these assays can be found in the supplemental Materials and methods.
Fluorescent labeling and confocal microscopy
BMSCs or 5T33MMvt cells were incubated in medium containing 1,1′-Dioctadecyl-3,3,3′,3′-Tetramethylindodicarbocyanine (DID [red]) (red) cell-labeling solution for 20 minutes at 37°C and washed with medium 3 times. Exosome suspension was labeled with DIO (green) and repelleted, using ExoQuick-TC exosome precipitation solution, 3 times. Cellular nuclei were stained using 4,6 diamidino-2-phenylindole. After incubation of DIO-labeled exosomes with DID-labeled cells, images of exosome uptake were acquired with a confocal laser scanning microscope LSM 710, using ZEN software (Carl Zeiss).
Mouse cytokine array
Exosomal proteins were extracted using lysis buffer supplemented with a protease and phosphatase inhibitor cocktail. Using the Mouse Cytokine Array Panel A kits (R&D Systems), 200 μg exosomal protein was tested for cytokine levels, according to the manufacturer’s instructions. Briefly, exosome lysates were mixed with the detection antibody cocktail and incubated with the membrane that contains 40 different anticytokine capture antibodies overnight at 4°C. After incubation with Streptavidin-HRP, the membranes were incubated with chemiluminescent substrate and exposed to X-ray film. The pixel densities of proteins were quantified using ImageJ 1.47 software.
Tracking of the transplanted cells or exosomes
5T33MMvv cells were stained with 3.5 μg/mL DIR (Invitrogen) for 30 minutes and washed 3 times with PBS. Next, 2.5 × 106 DIR-labeled 5T33MM cells in PBS were intravenously injected into naive C57BL/KaLwRij mice. Twenty-four hours after injection, the mice were killed and prepared for in vivo and ex vivo imaging. Naive or 5T33 BMSC exosome suspensions were labeled with DIR and repelleted, using ExoQuick-TC exosome precipitation solution, 3 times. Next, 500 μg DIR-labeled exosomes were intravenously injected in 5T33MM mice for 24 hours. The distributions of labeled 5T33MMvv or BMSC-derived exosomes in mice were detected using the Fluobeam800 Imaging System (Fluoptics).
Statistical analysis
Statistical significance was determined using the Mann-Whitney test or 1-way analysis of variance; P < .05 was regarded as statistically significant.
Results
BMSCs and MM cells communicate with each other through exosomes
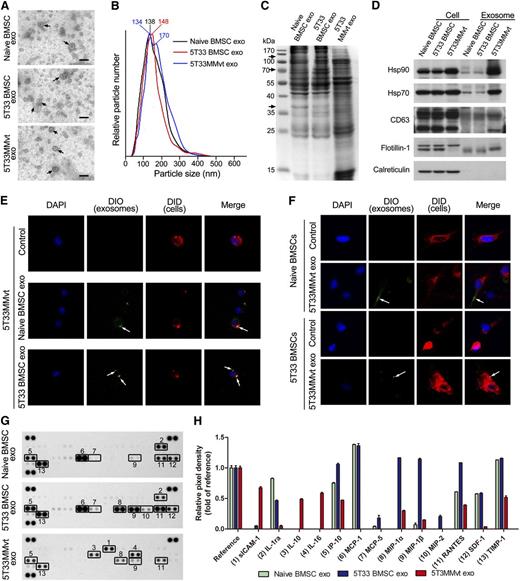
We first examined whether BMSCs and 5T33MMvt cells secrete exosomes. Therefore, primary BMSCs obtained from naive mice, 5T33MM mice, or 5T33MMvt cells were cultured in serum-free medium for 48 hours, and the exosomes were isolated from the conditioned medium. The size of the isolated exosomes was confirmed using transmission electron microscopy, and typical cup-shaped membrane particles with a diameter of 50 to 80 nm were observed (Figure 1A). NTA showed a size distribution of 50 to 250 nm for the 3 types of exosomes (Figure 1B). Furthermore, Coomassie blue-stained sodium dodecyl sulfate-polyacrylamide gel electrophoresis gels showed different protein electrophoresis patterns between BMSC- and MM-derived exosomes and differences in the protein bands between the exosomes obtained from naive and 5T33 BMSCs (Figure 1C). Several exosomal markers including heat shock protein 90 (Hsp90), Hsp70, CD63, and flotillin-1 were detected in these exosomes. Calreticulin, an intracellular contaminant, was negative (Figure 1D). Next, we examined whether the exosomes secreted from BMSCs and MM cells could be taken up by each other. For this purpose, DID-labeled 5T33MMvt cells (Figure 1E) or BMSCs (Figure 1F) were incubated with DIO-labeled BMSC- or 5T33MMvt cell-derived exosomes, respectively, for 2 hours, and exosome uptake by MM cells or BMSCs was observed. For excluding the dye contamination in DIO-labeled exosomes, DIO dye was treated in the same way as exosomes and considered a negative control. No green fluorescence was detected when the 5T33MMvt cells were treated with this control medium (supplemental Figure 1). Protein content of the exosomes was also determined, using a cytokine array (Figure 1G-H), and the results showed that the composition of the exosomes obtained from BMSCs or MM cells is different. These results indicate that BMSCs and MM cells can communicate with each other and exchange cytokines through exosome secretion and uptake.
Exosome mediate communication between BMSCs and MM cells. (A) Transmission electron microscopy images of exosomes derived from BMSCs and 5T33MMvt cells. The scale bars indicate 100 nm, and the arrows indicate the typical exosomes. (B) Size distributions of BMSC- and MM cell-derived exosomes were identified using the NTA. (C) Coomassie blue-stained sodium dodecyl sulfate-polyacrylamide gel electrophoresis gel after separation of total lysates from naive BMSC, 5T33 BMSC-derived, and 5T33MMvt cell-derived exosomes. Arrows indicate different protein bands between naive and 5T33 BMSC-derived exosomes. (D) Exosomal positive markers Hsp90, Hsp70, CD63, and flotillin-1 were detected in naive, 5T33 BMSC-derived, and 5T33MMvt cell-derived exosomes using western blot, whereas negative marker calreticulin was not. (E) DID-labeled 5T33MMvt cells (red) were incubated with DIO-labeled naive or 5T33 BMSC-derived exosomes (100 μg/mL, green) for 2 hours, and the exosome uptake by MM cells was detected (original magnification, ×740). Arrows indicate the fusion of the membrane. (F) DID-labeled naive or 5T33 BMSCs (red) were incubated with DIO-labeled 5T33MMvt exosomes (100 μg/mL, green) for 2 hours, and the exosome uptake by BMSCs was detected (original magnification, ×740). Arrows indicate the fusions of the membrane. (G) Cytokines in BMSC-derived and 5T33MMvt cell-derived exosomes were analyzed using a mouse cytokine array. (H) The pixel densities of proteins in cytokine array were quantified and presented as the fold of reference.
Exosome mediate communication between BMSCs and MM cells. (A) Transmission electron microscopy images of exosomes derived from BMSCs and 5T33MMvt cells. The scale bars indicate 100 nm, and the arrows indicate the typical exosomes. (B) Size distributions of BMSC- and MM cell-derived exosomes were identified using the NTA. (C) Coomassie blue-stained sodium dodecyl sulfate-polyacrylamide gel electrophoresis gel after separation of total lysates from naive BMSC, 5T33 BMSC-derived, and 5T33MMvt cell-derived exosomes. Arrows indicate different protein bands between naive and 5T33 BMSC-derived exosomes. (D) Exosomal positive markers Hsp90, Hsp70, CD63, and flotillin-1 were detected in naive, 5T33 BMSC-derived, and 5T33MMvt cell-derived exosomes using western blot, whereas negative marker calreticulin was not. (E) DID-labeled 5T33MMvt cells (red) were incubated with DIO-labeled naive or 5T33 BMSC-derived exosomes (100 μg/mL, green) for 2 hours, and the exosome uptake by MM cells was detected (original magnification, ×740). Arrows indicate the fusion of the membrane. (F) DID-labeled naive or 5T33 BMSCs (red) were incubated with DIO-labeled 5T33MMvt exosomes (100 μg/mL, green) for 2 hours, and the exosome uptake by BMSCs was detected (original magnification, ×740). Arrows indicate the fusions of the membrane. (G) Cytokines in BMSC-derived and 5T33MMvt cell-derived exosomes were analyzed using a mouse cytokine array. (H) The pixel densities of proteins in cytokine array were quantified and presented as the fold of reference.
BMSC-derived exosomes increased MM cell migration and can transfer to the BM in vivo
Because several chemotactic proteins such as monocyte chemoattractant protein 1 (MCP-1), interferon-inducible protein 10 (IP-10), and stromal cell-derived factor 1 (SDF-1) were detected in BMSC-derived exosomes, we next determined whether these exosomes promote MM cell migration, using a transwell system. Both naive and 5T33 BMSC exosomes increased the migration of 5T33MMvt and 5T33MMvv cells in a dose-dependent manner (Figure 2A). An antagonist of CXCR4 (AMD3100) and CC chemokine receptor 2 (CCR2) that inhibits SDF-1-mediated and MCP-1-mediated chemotaxis were used to confirm the involvement of these 2 chemokines in exosome-induced migration. AMD3100 and the CCR2 antagonist significantly decreased exosome-induced migration in 5T33MMvt cells (Figure 2B). These 2 antagonists also inhibited the exosome-induced migration in 5T33MMvv cells; however, the differences were smaller and not significant (Figure 2C). One of the key features of MM is the localization of the MM cells in the BM. The homing process of MM cells toward the BM is partly a result of the attraction by BMSCs. We confirmed that 5T33MMvv cells homed to the BM, spleen, and liver 1 day after intravenous injection in naive mouse (Figure 2D). Both naive and 5T33 BMSC-derived exosomes were also localized in the BM, spleen, and liver in the 5T33MM model after intravenous injection (Figure 2E).
BMSC-derived exosomes increase migration of MM cells and home to the BM in vivo. (A) 5T33MMvt or 5T33MMvv cells were incubated with 40 or 80 μg/mL naive or 5T33 BMSC-derived exosomes in transwell system, and transmigrated MM cells were counted using fluorescence-activated cell sorter. The results are presented as the percentage of total MM cells in the transwell system. 5T33MMvt (B) and 5T33MMvv (C) cells pretreated with AMD3100 (50 μM) or CCR2 antagonist (100 nM) for 90 minutes were incubated with 80 μg/mL naive or 5T33 BMSC-derived exosomes in transwell system for another 2 hours. Transmigrated MM cells were counted using fluorescence-activated cell sorter, and the results are presented as percentage of the total MM cells in the transwell system. Mean values ± standard deviation for 3 independent experiments are shown. *P < .05; **P < .01; ***P < .001. (D) Here, 2.5 × 106 DIR-labeled 5T33MMvv cells were intravenously injected into naive mice. At 24 hours after injection, the localization of MM cells was detected using whole-body imaging. The distribution of 5T33MMvv cells in organs was also measured using an imaging system. The fluorescence intensity scale is displayed on the right side of the images. (E) Five hundred micrograms DIR-labeled naive or 5T33 BMSC-derived exosomes were intravenously injected in 5T33 mice for 24 hours. The localization of the exosomes in organs was detected as in C. The fluorescence intensity scale is indicated on the right side of the images. The highest intensity is marked as 100% and indicated by red color, whereas the lowest is indicated by dark blue. Arrows indicate the organs in vivo. Le, Legs; Li, liver, Sc, spinal column; Sp, spleen.
BMSC-derived exosomes increase migration of MM cells and home to the BM in vivo. (A) 5T33MMvt or 5T33MMvv cells were incubated with 40 or 80 μg/mL naive or 5T33 BMSC-derived exosomes in transwell system, and transmigrated MM cells were counted using fluorescence-activated cell sorter. The results are presented as the percentage of total MM cells in the transwell system. 5T33MMvt (B) and 5T33MMvv (C) cells pretreated with AMD3100 (50 μM) or CCR2 antagonist (100 nM) for 90 minutes were incubated with 80 μg/mL naive or 5T33 BMSC-derived exosomes in transwell system for another 2 hours. Transmigrated MM cells were counted using fluorescence-activated cell sorter, and the results are presented as percentage of the total MM cells in the transwell system. Mean values ± standard deviation for 3 independent experiments are shown. *P < .05; **P < .01; ***P < .001. (D) Here, 2.5 × 106 DIR-labeled 5T33MMvv cells were intravenously injected into naive mice. At 24 hours after injection, the localization of MM cells was detected using whole-body imaging. The distribution of 5T33MMvv cells in organs was also measured using an imaging system. The fluorescence intensity scale is displayed on the right side of the images. (E) Five hundred micrograms DIR-labeled naive or 5T33 BMSC-derived exosomes were intravenously injected in 5T33 mice for 24 hours. The localization of the exosomes in organs was detected as in C. The fluorescence intensity scale is indicated on the right side of the images. The highest intensity is marked as 100% and indicated by red color, whereas the lowest is indicated by dark blue. Arrows indicate the organs in vivo. Le, Legs; Li, liver, Sc, spinal column; Sp, spleen.
BMSC-derived exosomes promote MM cell viability and proliferation
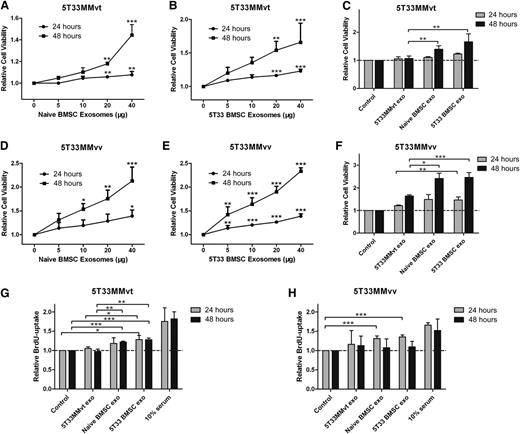
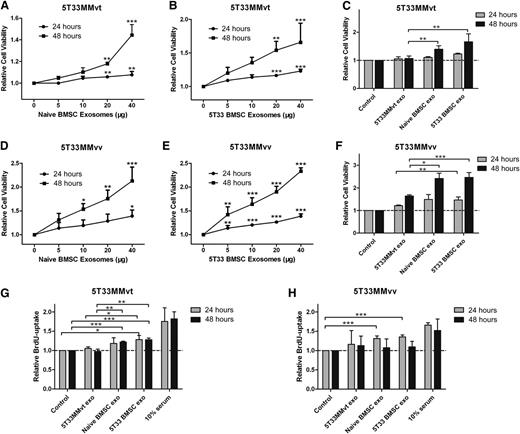
It is well known that BMSCs increase the viability and proliferation of MM cells;1,24,25 however, the role of exosomes in these actions is not clear. Here, we examined the effects of treating 5T33MMvt and 5T33MMvv cells with BMSC-derived exosomes on cell viability and proliferation. Naive (Figure 3A,D) and 5T33 (Figure 3B,E) BMSC-derived exosomes significantly increased 5T33MMvt and 5T33MMvv cell viability in a time- and dose-dependent manner. We next used the exosomes derived from 5T33MMvt cells as a control to confirm whether the increase we detected was a common effect of exosomes. BMSC-derived exosomes increased 5T33MMvt and 5T33MMvv cell viability compared with exosomes obtained from 5T33MMvt cells (Figure 3C,F). However, no difference in MM cell viability was observed between treatment with naive and treatment with 5T33 BMSC-derived exosomes (Figure 3C,F). Cell proliferation of 5T33MMvt (Figure 3G) and 5T33MMvv (Figure 3H) was also evaluated, using a BrdU-uptake assay. As expected, proliferation was increased after treatment with both naive and 5T33 BMSC exosomes. These data demonstrate the involvement of exosomes in BMSC-induced promotion of MM cell viability and proliferation.
BMSC-derived exosomes increase MM cell viability and proliferation. 5T33MMvt (A,B) or 5T33MMvv (D,E) cells in serum-free medium were incubated with indicated amounts of naive (A,D) or 5T33 (B,E) BMSC-derived exosomes for 24 or 48 hours, and cell viability was measured using a luminescent cell viability assay. 5T33MMvt (C) or 5T33MMvv (F) cells in serum-free medium were treated with 40 μg/mL naive or 5T33 BMSC-derived or 5T33MMvt cell-derived exosomes for 24 or 48 hours, and cell viability was determined. 5T33MMvt (G) or 5T33MMvv (H) cells in serum-free medium were treated with 40 μg/mL naive or 5T33 BMSC-derived or 5T33MMvt cell-derived exosomes for 24 or 48 hours, and cell proliferation was measured using a BrdU cell proliferation assay. Ten percent serum was used as a positive control. Mean values ± standard deviation for 3 independent experiments are shown. *P < .05; **P < .01; ***P < .001.
BMSC-derived exosomes increase MM cell viability and proliferation. 5T33MMvt (A,B) or 5T33MMvv (D,E) cells in serum-free medium were incubated with indicated amounts of naive (A,D) or 5T33 (B,E) BMSC-derived exosomes for 24 or 48 hours, and cell viability was measured using a luminescent cell viability assay. 5T33MMvt (C) or 5T33MMvv (F) cells in serum-free medium were treated with 40 μg/mL naive or 5T33 BMSC-derived or 5T33MMvt cell-derived exosomes for 24 or 48 hours, and cell viability was determined. 5T33MMvt (G) or 5T33MMvv (H) cells in serum-free medium were treated with 40 μg/mL naive or 5T33 BMSC-derived or 5T33MMvt cell-derived exosomes for 24 or 48 hours, and cell proliferation was measured using a BrdU cell proliferation assay. Ten percent serum was used as a positive control. Mean values ± standard deviation for 3 independent experiments are shown. *P < .05; **P < .01; ***P < .001.
BMSC-derived exosomes facilitate MM cell survival
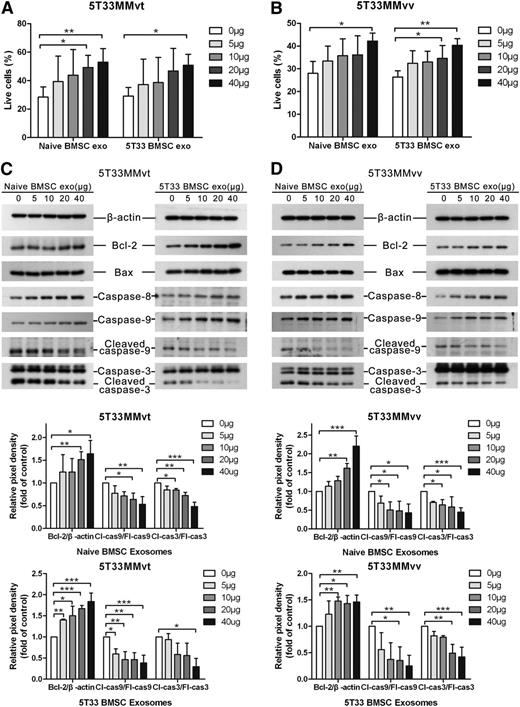
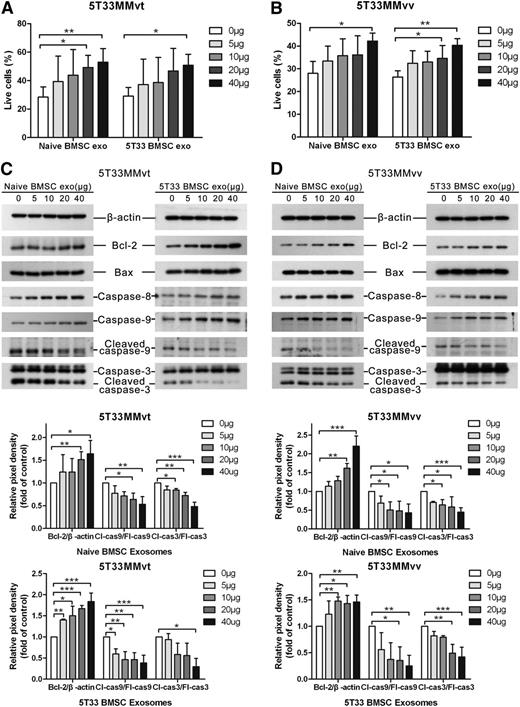
As BMSC-derived exosomes increased the viability of MM cells, we wished to determine whether this was solely because of an increase in cell proliferation or was also because of an increase in survival. Thus, the apoptosis of MM cells was measured after the treatment with BMSC-derived exosomes. Both naive and 5T33 BMSC-derived exosomes increased cell survival of 5T33MMvt (Figure 4A) and 5T33MMvv (Figure 4B) cells in a dose-dependent manner under serum-free conditions. High doses (100 and 200 μg/mL) of BMSC-derived exosomes increased the live 5T33MMvt (supplemental Figure 2A) and 5T33MMvv (supplemental Figure 2B) cells up to 70% and 60%, respectively. Meanwhile, apoptosis-related proteins Bcl-2, Bax, caspase-8, caspase-9, and caspase-3 were also determined in 5T33MMvt (Figure 4C) and 5T33MMvv (Figure 4D) cells, using western blot. Antiapoptotic protein Bcl-2 was increased after the treatment with both naive and 5T33 BMSC-derived exosomes, whereas proapoptotic protein Bax was not changed. Full-length caspase-8 and caspase-9 were gradually increased when the MM cells were treated with increased doses of exosomes. Cleaved caspase-9 and caspase-3 were gradually reduced after the treatment with increased amounts of BMSC-derived exosomes, suggesting that BMSCs inhibit the activation of caspase-9 and caspase-3 to promote MM cell survival.
BMSC-derived exosomes increase MM cell survival and reduce the activation of caspase-3/8/9. 5T33MMvt (A) or 5T33MMvv (B) cells were cultured in serum-free conditions and treated with different amounts of naive or 5T33 BMSC-derived exosomes for 24 (5T33MMvv cells) or 48 hours (5T33MMvt cells). Apoptotic cells were determined using 7-aminoactinomycin D (7-AAD) and Annexin-V staining after treatment. A similar set-up was used to detect apoptosis-related proteins Bcl-2, Bax, caspase-8, caspase-9, and caspase-3 in 5T33MMvt (C) and 5T33MMvv (D) cells after exosome treatment, using western blot. The analysis of β-actin protein was included as a loading control. The pixel densities of proteins were quantified from 3 independent experiments and presented by histograms in the bottom panel. Mean values ± standard deviation for 3 independent experiments are shown. *P < .05; **P < .01; ***P < .001. cas3, caspase-3; cas9, caspase-9; Cl, cleaved; Fl, full-length.
BMSC-derived exosomes increase MM cell survival and reduce the activation of caspase-3/8/9. 5T33MMvt (A) or 5T33MMvv (B) cells were cultured in serum-free conditions and treated with different amounts of naive or 5T33 BMSC-derived exosomes for 24 (5T33MMvv cells) or 48 hours (5T33MMvt cells). Apoptotic cells were determined using 7-aminoactinomycin D (7-AAD) and Annexin-V staining after treatment. A similar set-up was used to detect apoptosis-related proteins Bcl-2, Bax, caspase-8, caspase-9, and caspase-3 in 5T33MMvt (C) and 5T33MMvv (D) cells after exosome treatment, using western blot. The analysis of β-actin protein was included as a loading control. The pixel densities of proteins were quantified from 3 independent experiments and presented by histograms in the bottom panel. Mean values ± standard deviation for 3 independent experiments are shown. *P < .05; **P < .01; ***P < .001. cas3, caspase-3; cas9, caspase-9; Cl, cleaved; Fl, full-length.
p38, p53, c-Jun N-terminal kinase, and Akt pathways in MM cells are influenced by BMSC-derived exosome
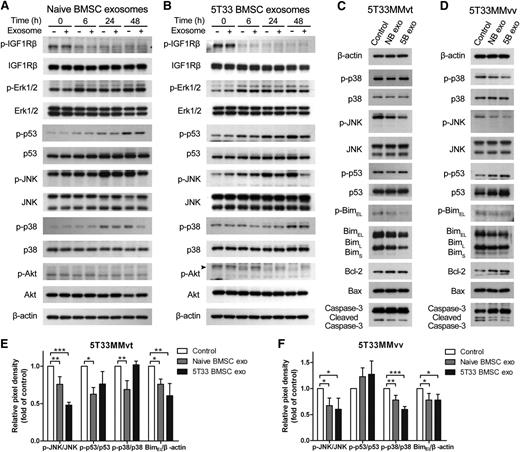
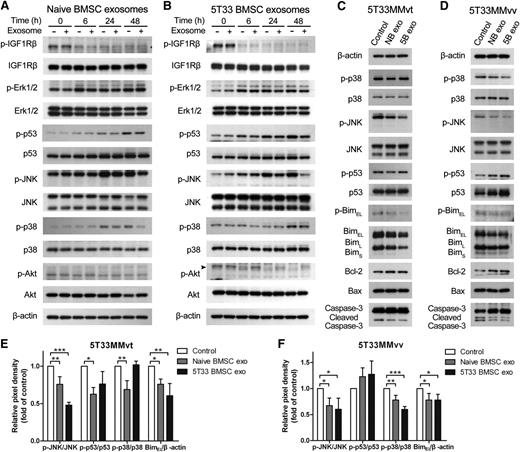
As we determined that exosomes are involved in MM cell proliferation and survival, we next investigated which pathways are influenced by exosomes. Several pathways related to cell survival were investigated in 5T33MMvt cells after treatment with BMSC-derived exosomes. Naive and 5T33 BMSC-derived exosomes reduced phosphorylated c-Jun N-terminal kinase (JNK) in 5T33MMvt cells after 48 hours of treatment, and a smaller decrease of phosphorylated p53 and p38 was also observed at 48 hours with naive exosomes (Figure 5A-B). However, there was no clear inhibition of phosphorylated p38 by 5T33 BMSC-derived exosomes (Figure 5B). We did not see changes in phosphorylated insulin-like growth factor 1 receptor β, Erk1/2, and Akt when 5T33MMvt cells were treated with naive BMSC-derived exosomes at short (supplemental Figure 3) or long (Figure 5A) time points. 5T33 BMSC-derived exosomes did prevent the decrease in phosphorylation of Akt, induced by serum starvation in 5T33MMvt cells (Figure 5B). We then compared the effects of naive and 5T33 BMSC-derived exosomes on 5T33MMvt (Figure 5C) and 5T33MMvv cells (Figure 5D) at 24 hours and quantified the protein changes in Figure 5E-F. We can see that p-JNK is inhibited more by 5T33 BMSC-derived exosomes in both cell types but that the effect on p38 is more pronounced in 5T33MMvv cells. Intriguingly, the opposite effects on p53 expression were found, with an increase in 5T33MMvv cells (P > .05; Figure 5F) and a decrease in 5T33MMvt cells (Figure 5E). The downstream proteins of the apoptosis pathway, including Bim, Bcl-2, Bax, and caspase-3, were further detected in 5T33MMvt and 5TMMvv cells after 24 hours of exosome treatment. In both cell types, we found a reduction in (p)-BimEL by naive and 5T33 BMSC-derived exosomes and an increase in the expression of the antiapoptotic protein Bcl-2 and reduced cleavage of caspase-3 (Figure 5C-F).
p38, p53, JNK, and Akt pathways are influenced by BMSC-derived exosome. 5T33MMvt cells in serum-free medium were treated with or without 40 μg/mL naive (A) or 5T33 (B) BMSC-derived exosomes for indicated times. Expressions of total and phosphorylated insulin-like growth factor 1 receptor β, Erk1/2, p53, JNK, p38, and Akt in MM cells were determined using western blot analysis. 5T33MMvt (C,E) or 5T33MMvv (D,F) cells were treated with 40 μg/mL naive or 5T33 BMSC-derived (NB or 5B) exosomes in serum-free conditions for 24 hours. The total and phosphorylated p53, JNK, p38, and Bim and the expression of Bcl-2, Bax, and caspase-3 were detected using western blot. The analysis of β-actin protein was included as a loading control. The pixel densities of p53, p38, BimEL, and JNK in 5T33MMvt (E) or 5T33MMvv (F) cells after 24 hours of treatment were quantified from 3 independent experiments. The pixel density of phosphorylations of p53, p38, and JNK were normalized to their total proteins. The expression of BimEL was normalized to β-actin, and all these results were presented by histograms. Mean values ± standard deviation for 3 independent experiments are shown. *P < .05; **P < .01; ***P < .001.
p38, p53, JNK, and Akt pathways are influenced by BMSC-derived exosome. 5T33MMvt cells in serum-free medium were treated with or without 40 μg/mL naive (A) or 5T33 (B) BMSC-derived exosomes for indicated times. Expressions of total and phosphorylated insulin-like growth factor 1 receptor β, Erk1/2, p53, JNK, p38, and Akt in MM cells were determined using western blot analysis. 5T33MMvt (C,E) or 5T33MMvv (D,F) cells were treated with 40 μg/mL naive or 5T33 BMSC-derived (NB or 5B) exosomes in serum-free conditions for 24 hours. The total and phosphorylated p53, JNK, p38, and Bim and the expression of Bcl-2, Bax, and caspase-3 were detected using western blot. The analysis of β-actin protein was included as a loading control. The pixel densities of p53, p38, BimEL, and JNK in 5T33MMvt (E) or 5T33MMvv (F) cells after 24 hours of treatment were quantified from 3 independent experiments. The pixel density of phosphorylations of p53, p38, and JNK were normalized to their total proteins. The expression of BimEL was normalized to β-actin, and all these results were presented by histograms. Mean values ± standard deviation for 3 independent experiments are shown. *P < .05; **P < .01; ***P < .001.
BMSC-derived exosomes induce drug resistance in MM cells
It is known that BMSCs contribute to drug resistance of MM cells through cell-to-cell contact or secretion of cytokines. In this study, we examined whether BMSC-derived exosomes contribute to this drug resistance. We used several clinically relevant drugs for MM, including a histone deacetylase inhibitor JNJ-26481585,23 a DNA synthesis inhibitor Doxorubicin, and a proteasome inhibitor bortezomib (Btz), to examine the drug resistance of MM cells after BMSC-derived exosome treatment. BMSC-derived exosomes significantly increased MM cell viability in the presence of Btz in 5T33MMvt cells (Figure 6A). We next tested the drug resistance to Btz after exosome treatment in 5T33MMvv cells. Similar to 5T33MMvt cells, 5T33MMvv cells showed drug resistance to Btz in the presence of BMSC-derived exosomes (Figure 6B). When studying apoptosis, BMSC-derived exosomes significantly increased percentages of living 5T33MMvt (Figure 6C) and 5T33MMvv cells (Figure 6D) in the presence of Btz. Apoptosis-related proteins Bcl-2, Bax, caspase-8, caspase-9, caspase-3, and PARP were measured in 5T33MMvt cells after treatment with BMSC-derived exosomes in the absence or presence of Btz. BMSC-derived exosomes could block the significant reduction of Bcl-2 expression caused by Btz and reduced cleaved caspase-9, caspase-3, and PARP in the absence or presence of Btz (Figure 6E). In the presence of Btz, more full-length caspase-8, caspase-9, capase-3, and PARP were detected in 5T33MMvt cells after incubation with BMSC-derived exosomes. These data indicate the involvement of exosomes in BMSC-induced drug resistance in MM cells.
BMSC-derived exosomes induce drug resistance of MM cells to bortezomib. (A) 5T33MMvt cells in serum medium were treated with naive or 5T33 BMSC-derived exosomes (40 μg/mL) in the absence or presence of JNJ (20 nM) and Dox (4 μM), and indicated concentration of Btz for 48 hours and cell viability was measured. (B) 5T33MMvv cells in serum medium were cultured with naive or 5T33 BMSC-derived exosomes (40 μg/mL) in the absence or presence of Btz for 24 hours, and cell viability was evaluated. (C) 5T33MMvt cells in serum medium were treated with naive or 5T33 BMSC-derived (40 μg/mL) exosomes in the absence or presence of different concentration of Btz for 48 hours, and the percentage of living cells was determined using fluorescence-activated cell sorter staining. (D) 5T33MMvv cells in serum medium were treated with naive or 5T33 BMSC-derived exosomes (40 μg/mL) in the absence or presence of Btz (1.75nM), and the percentage of living cells was measured using fluorescence-activated cell sorter staining. (E) 5T33MMvt cells in serum medium were treated with naive or 5T33 BMSC-derived exosomes (40 μg/mL) in the absence or presence of Btz (2 nM), and apoptosis-related proteins Bcl-2, Bax, caspase-8, caspase-9, caspase-3, and PARP were detected using western blot. The pixel densities of the proteins were quantified from 3 independent experiments and presented by histograms in the right panel. Mean values ± standard deviations for 3 independent experiments are shown.*P < .05; **P < .01; ***P < .001. Cl, cleaved; Fl, full-length; cas-3, caspase-3; cas-8, caspase-8; cas-9, caspase-9.
BMSC-derived exosomes induce drug resistance of MM cells to bortezomib. (A) 5T33MMvt cells in serum medium were treated with naive or 5T33 BMSC-derived exosomes (40 μg/mL) in the absence or presence of JNJ (20 nM) and Dox (4 μM), and indicated concentration of Btz for 48 hours and cell viability was measured. (B) 5T33MMvv cells in serum medium were cultured with naive or 5T33 BMSC-derived exosomes (40 μg/mL) in the absence or presence of Btz for 24 hours, and cell viability was evaluated. (C) 5T33MMvt cells in serum medium were treated with naive or 5T33 BMSC-derived (40 μg/mL) exosomes in the absence or presence of different concentration of Btz for 48 hours, and the percentage of living cells was determined using fluorescence-activated cell sorter staining. (D) 5T33MMvv cells in serum medium were treated with naive or 5T33 BMSC-derived exosomes (40 μg/mL) in the absence or presence of Btz (1.75nM), and the percentage of living cells was measured using fluorescence-activated cell sorter staining. (E) 5T33MMvt cells in serum medium were treated with naive or 5T33 BMSC-derived exosomes (40 μg/mL) in the absence or presence of Btz (2 nM), and apoptosis-related proteins Bcl-2, Bax, caspase-8, caspase-9, caspase-3, and PARP were detected using western blot. The pixel densities of the proteins were quantified from 3 independent experiments and presented by histograms in the right panel. Mean values ± standard deviations for 3 independent experiments are shown.*P < .05; **P < .01; ***P < .001. Cl, cleaved; Fl, full-length; cas-3, caspase-3; cas-8, caspase-8; cas-9, caspase-9.
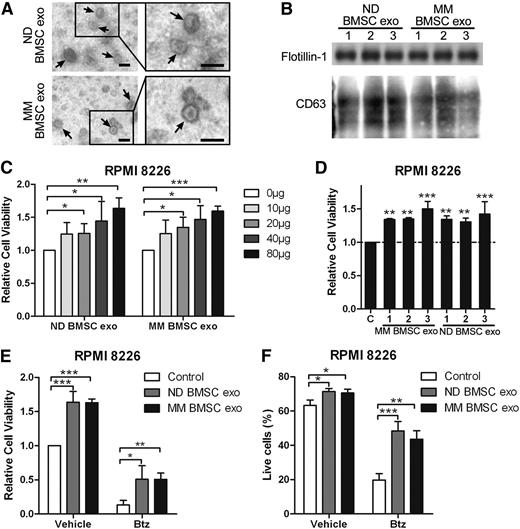
Human BMSC-derived exosomes promote MM cell survival and induce drug resistance
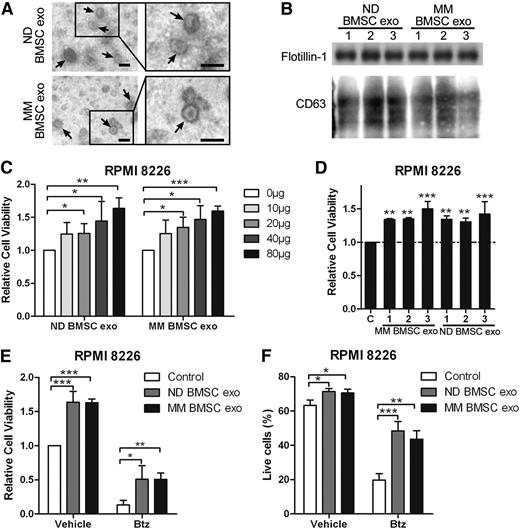
As we demonstrated the ability of BMSC-derived exosomes to promote MM cell survival and drug resistance in murine cells, we next wanted to confirm these findings using human cells. Size and markers of exosomes isolated from normal donors’ and MM patients’ (respectively, normal donor [ND] and MM patients) BMSCs were confirmed (Figure 7A-B). ND and MM BMSC-derived exosomes increased the viability of RPMI8226 cells dose-dependently (Figure 7C). The effect of 3 different ND and 3 MM BMSC-derived exosome samples on MM cell viability were similar (Figure 7D). In addition, both ND and MM BMSC-derived exosomes significantly increased cell viability in the presence of Btz (Figure 7E). ND and MM BMSC-derived exosomes increased the percentage of living cells by about 25% in the presence of Btz, whereas they increased living cells by about 9% in the absence of Btz (Figure 7F). These results confirm the crucial role of BMSC-derived exosome in MM cell survival and drug resistance.
Human BMSC-derived exosomes increase the MM cell viability and induce drug resistance to bortezomib. (A) Transmission electron microscopy images of exosomes derived from ND and MM patients’ BMSCs. The scale bars indicate 100 nm, and the arrows indicate the typical exosomes. (B) Exosomal positive markers CD63 and flotillin-1 were detected in ND and MM BMSC-derived exosomes using western blot. (C) RPMI 8226 cells in serum-free medium were treated with different amounts of ND or MM BMSC-derived exosomes for 48 hours, and cell viability was measured and presented as the fold of control. (D) RPMI 8226 cells in serum-free medium were cultured with 40 μg/mL exosomes derived from 3 ND BMSCs or MM BMSCs for 48 hours, and cell viability was measured. (E) RPMI 8226 cells were cultured in serum-free medium with 80 μg/mL ND or MM BMSC-derived exosomes in the absence or presence of Btz (2 nM) for 48 hours, and cell viability was measured. (F) RPMI 8226 cells in serum-free medium were treated with ND or MM BMSC-derived exosomes (80 μg/mL) in the absence or presence of Btz (2 nM), and the live cells were measured using fluorescence-activated cell sorter staining. Mean values ± standard deviation for 3 independent experiments are shown. *P < .05; **P < .01; ***P < .001.
Human BMSC-derived exosomes increase the MM cell viability and induce drug resistance to bortezomib. (A) Transmission electron microscopy images of exosomes derived from ND and MM patients’ BMSCs. The scale bars indicate 100 nm, and the arrows indicate the typical exosomes. (B) Exosomal positive markers CD63 and flotillin-1 were detected in ND and MM BMSC-derived exosomes using western blot. (C) RPMI 8226 cells in serum-free medium were treated with different amounts of ND or MM BMSC-derived exosomes for 48 hours, and cell viability was measured and presented as the fold of control. (D) RPMI 8226 cells in serum-free medium were cultured with 40 μg/mL exosomes derived from 3 ND BMSCs or MM BMSCs for 48 hours, and cell viability was measured. (E) RPMI 8226 cells were cultured in serum-free medium with 80 μg/mL ND or MM BMSC-derived exosomes in the absence or presence of Btz (2 nM) for 48 hours, and cell viability was measured. (F) RPMI 8226 cells in serum-free medium were treated with ND or MM BMSC-derived exosomes (80 μg/mL) in the absence or presence of Btz (2 nM), and the live cells were measured using fluorescence-activated cell sorter staining. Mean values ± standard deviation for 3 independent experiments are shown. *P < .05; **P < .01; ***P < .001.
BM samples were collected at the Myeloma Center Brussels for routine diagnostic or evaluation purposes after patients’ informed consent and in accordance with the Declaration of Helsinki. All research was approved by the local ethical committee (B.U.N. 143201316382).
Animals were treated and housed following the conditions approved by the Ethical Committee for animal experiments, Vrije Universiteit Brussel (license no. LA1230281). Approval for these specific experiments was obtained by the Committee (approval no. 14-281-4).
Discussion
In recent years, accumulating evidence indicates that exosomes mediate cell-to-cell communication26-28 and confirms the crucial functions of exosomes in hematological malignancies. These studies have shown the involvement of exosomes in cell proliferation,29,30 angiogenesis,31,32 differentiation,29 natural killer cell cytotoxicity,33 and induction of antitumor T cell immunity.34 However, the role of exosomes in MM pathogenesis and progression has not yet been thoroughly investigated. BMSCs in the BM microenvironment interact with MM cells through cell-to-cell contact, releasing cytokines and other functional components to support tumor cell growth. Nevertheless, the function of the newfound communicator, the exosome, in these alterations remains largely unknown. Our present study provides detailed and novel evidence of the involvement of BMSC-derived exosomes in BMSC-induced MM cell growth, survival, and drug resistance, leading to a better understanding of the interactions between exosomes in the BM microenvironment and the MM cells.
Exosomes have been purified using several strategies and techniques, including ultracentrifugation, density gradient separation, immunoaffinity capture, and ExoQuick precipitation. Modified exosome precipitation using ExoQuick solution has been proven to be a simple and efficient method compared with the standard method of ultracentrifugation and 30% sucrose cushion.35 In the present study, we isolated exosomes using ExoQuick solution, and the morphology and markers of exosomes were confirmed on the basis of electron microscopy and western blot. The size distribution of exosomes of 50 to 250 nm was assessed using NTA; these values are larger than what we observed from TEM images. This discordance is partly a result of the lower sensitivity of NTA in the range of 20 to 60 nm because the smaller material in this range was seen by electron microscopy, but not by NTA.36 Many studies have shown a similar size distribution of exosomes compared with our result when using NTA.36-39 In addition, we found similar effects of BMSC-derived exosomes isolated using ExoQuick precipitation compared with OptiPrep density-based separation on MM cell viability (data not shown).
Exosomes mediate cell-to-cell communication through transfer of their contents. Here, we observed the exchange of exosomes between BMSCs or MM cells and demonstrated differences in cytokine profiles of BMSC- and 5T33MMvt cell-derived exosomes. Several cytokines such as interleukin 1ra (IL-1ra), MCP-1, macrophage inflammatory protein 1α, RANTES (regulated upon activation, normal T cell expressed and secreted), and SDF-1 were found in murine BMSC-derived exosomes. Similarly, it has been reported that human BMSC-derived exosomes contain IL-6, MCP-1, and IL-13.30 These data suggest that exosomes selectively carry certain cytokines and transfer them to the recipient cells. BMSCs produce many chemotactic proteins, such as MCP-1, MCP-2, MCP-3,40 SDF-1,41,42 and IGF-1,3,43 to trigger MM cell migration. Our results show that BMSC-derived exosomes carrying cytokines and chemokines induce migration of the 5T33MM cells. When SDF-1- and MCP-1-mediated chemotaxis were blocked by antagonists of their receptors, the migration of 5T33MMvt cells toward exosomes was completely inhibited. These antagonists only partially decreased the exosome-induced migration of 5T33MMvv, indicating that SDF-1 and MCP-1 are involved in exosome-induced migration, but that other chemokines cannot be excluded. 5T33MMvv cells are more sensitive to a variety of chemokines and cytokines, so they can still migrate despite the blocking of these 2 chemokines. Although these chemokines are wrapped in exosomes, there are 2 possible mechanisms to explain how exosomes can attract MM cells: the migrated MM cells take up the exosomes and release the chemokines within to attract more MM cells from the upper chamber of the transwell system, or on stimulation by the receptor or ligand on the exosome membrane, the migrated MM cells secrete more chemokines or cytokines to induce more cell migration. In vivo, we found that BMSC-derived exosomes were localized in the BM, spleen, and liver, similar to 5T33MM cells.
BMSCs produce growth factors to induce MM cell proliferation and survival by activating multiple growth and antiapoptotic signaling pathways.5,44,45 Our results show that both naive and 5T33 BMSC-derived exosomes also increased MM cell viability, proliferation, and survival, and that different pathways such as p38, p53, JNK, and Akt are involved in these actions. However, naive and 5T33 BMSC-derived exosomes promote cell survival through slightly different pathways and have different effects on 5T33MM vt and vv cells. This difference may be because of the different contents or membrane receptors and ligands on the exosomes and the different signaling machinery of the target cells. On the basis of a review from Camussi et al,8 we think BMSC-derived exosomes might induce cell survival by 3 different mechanisms: BMSC-derived exosomes could stimulate target cells through surface expressed receptors to induce signal transduction and the activation of antiapoptotic pathways in MM cells; BMSC-derived exosomes may transfer receptors to target cells, which could induce more signal transduction; and BMSC-derived exosomes could directly transfer transcription factors, as well as mRNA and miRNA, to MM cells and induce the activation of relevant pathways or the transcription of prosurvival genes. In 5T33MMvt and 5T33MMvv cells, both naive and 5T33 BMSC-derived exosomes inhibited the activation of the JNK pathway, as well as the expression and phosphorylation of Bim. Bim is a proapoptotic protein and is mainly regulated by the JNK pathway.46-48 Thus, promotion of MM cell survival likely results from the inhibition of the JNK pathway by BMSC-derived exosomes. A previous study has shown that IL-6 inhibits MM apoptosis via inhibition of the JNK/SAPK pathway.49 Inhibition of cleaved caspase-9 and caspase-3 was also found after the treatment with BMSC-derived exosomes. Serum starvation can induce the processing of full-length caspase-8,50,51 caspase-9,52-54 and caspase-3,52 and exosomes inhibit the cleavage of caspase-9 to protect these cells from apoptosis. Furthermore, the prosurvival effect of BMSC-derived exosomes was confirmed with exosomes of both ND and MM patients.
A study from Roccaro et al has shown that ND BM–mesenchymal stromal cells (BM-MSC)–derived exosomes decreased MM cell proliferation, whereas exosomes obtained from MM BM-MSC increased cell proliferation and facilitated MM progression.30 They found that this was because of a different miRNA and protein content between exosomes derived from normal and MM BM-MSCs. Our results confirmed a difference of exosome content, as we detected different cytokine patterns between exosomes derived from naive and 5T33 BMSCs. However, we did not observe different effects between these 2 exosome types or their human counterparts on MM cell proliferation and apoptosis. This discrepancy may be explained by the fact that we used total BMSCs, as this represents the BM microenvironment better compared with BM-MSCs. Furthermore, Roccaro et al investigated the role of exosomes, mainly using a xenograft MM mouse model. However, this xenograft model is not able to mimic the human BM microenvironment, which is crucial in MM progression. Our study demonstrates the multiple roles of BMSC-derived exosomes, using both human MM samples and the 5T33MM model, which provides a natural BM microenvironment for the MM cells.
Clinically relevant drugs such as bortezomib, lenalidomide, and thalidomide are widely used in the treatment of patients with MM; however, many patients still relapse and become resistant to these drugs.3 It is well known that the BM microenvironment and the interaction between BMSCs and MM cells contribute to drug resistance of these cells by cell-to-cell adhesion and secretion of soluble factors by BMSCs.24,55 As a consequence, several pathways related to drug resistance and cell survival such as Notch1,56,57 signal transducer and activator of transcription 3,58 Akt,5 and NF-κB59 are activated to protect the MM cells from death. Here we examined the effect of exosomes on bortezomib induced cell death. Bortezomib promotes MM cell death through induction of caspase-9, caspase-3, and PARP cleavage,60,61 and both naive and 5T33 BMSC-derived exosomes inhibited these effects to protect the MM cells from apoptosis. Our findings therefore reveal for the first time a novel mechanism in BMSC-induced drug resistance of MM cells to bortezomib through the secretion of exosomes.
In conclusion, the BM microenvironment provides an extremely intricate compartment to support MM growth, bone disease, and drug resistance. The reciprocal communication and interaction between BMSC and the MM cells play a pivotal role in this microenvironment. Here, our results highlight a novel communication mechanism between BMSCs and MM cells through exosome secretion and confirm that BMSC-derived exosome support MM development. These findings warrant further investigation into discovering ways to target these exosomes.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Carine Seynaeve, Eddy Himpe, and Marijke Baekeland for expert technical assistance.
This work was supported by Fonds voor Wetenschappelijk Onderzoek. J.W. was supported by a China Scholarship Council-Vrije Universiteit Brussel scholarship. E.M., E.V.V., and E.D.B. are postdoctoral fellows of Fonds voor Wetenschappelijk Onderzoek.
Authorship
Contribution: J.W., K.V., and E.M. conceived and designed the research and wrote the manuscript; J.W, E.M., A.H., S.H., and M.L. performed the experiment and analyzed the data; and A.H., S.H., M.L., E.V.V., E.D.B., T.L., O.D.W., E.M., and K.V. provided crucial suggestions and revised the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interest.
Correspondence: Eline Menu, Department of Hematology and Immunology-Myeloma Center Brussels, Vrije Universiteit Brussel, Laarbeeklaan 103, 1090 Brussels, Belgium; e-mail: eline.menu@vub.ac.be.
Reference
Author notes
K.V. and E.M. contributed equally to this study.