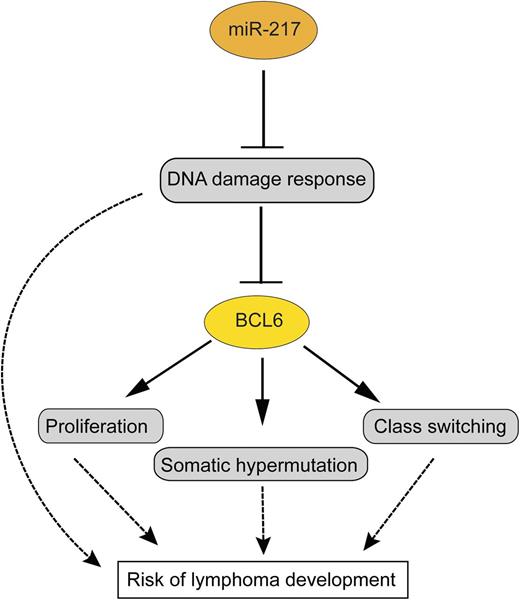
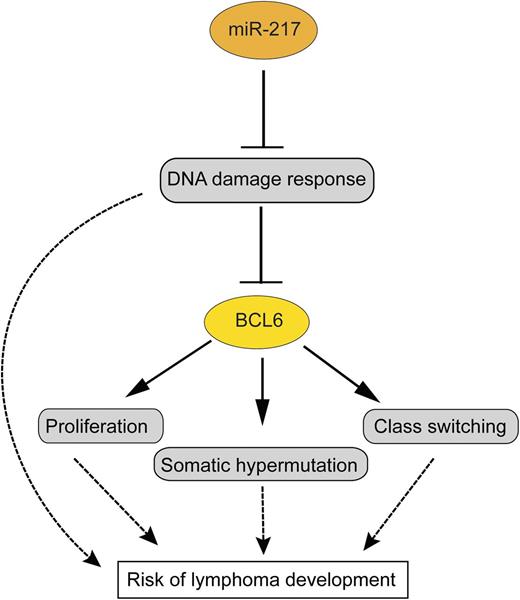
In this issue of Blood, de Yébenes et al identify miR-217 as a promoter of the germinal center reaction, partly by suppressing the DNA damage response. Overactivity of miR-217 is hence potentially dangerous, and, indeed, its uncontrolled expression causes lymphomas.1
miR-217 dampens the DNA damage response in germinal center B cells, and through this—and perhaps additional functions—promotes BCL6 expression and stabilization. This is presumably one of the main ways how miR-217 promotes germinal center B-cell proliferation, somatic hypermutation, and class switching. All these functions of miR-217 increase the risk for the development of B-cell lymphomas from germinal center B cells. Arrows indicate a positive influence; the T-shaped lines indicate an inhibitory effect.
miR-217 dampens the DNA damage response in germinal center B cells, and through this—and perhaps additional functions—promotes BCL6 expression and stabilization. This is presumably one of the main ways how miR-217 promotes germinal center B-cell proliferation, somatic hypermutation, and class switching. All these functions of miR-217 increase the risk for the development of B-cell lymphomas from germinal center B cells. Arrows indicate a positive influence; the T-shaped lines indicate an inhibitory effect.
MicroRNAs (miRNAs) have emerged as central posttranscriptional regulators of gene expression, involved in practically all aspects of cellular functions.2 miRNAs bind to mRNAs in a sequence-specific manner and inhibit their translation, often by causing mRNA degradation.2 One miRNA can have many different target mRNAs, which often hampers the identification of the specific functions of a miRNA.
The germinal center reaction is the central process in T cell-dependent humoral immune reactions, as clonal expansion of antigen-specific B cells, class-switch recombination of the immunoglobulin heavy chain constant region genes, somatic hypermutation of immunoglobulin V region genes, and the selection for high-affinity B-cell receptors takes plays in germinal centers.3 Germinal centers are also the structures where memory B cells and long-lived plasma cells are generated from positively selected B cells.3 Because of these major differentiation processes taking place in germinal center B cells, and as germinal center B cells show extensive changes in gene expression in comparison with pregerminal center naive B cells, it is to be expected that miRNAs play essential roles in these processes. However, thus far, only a few miRNAs with specific functions in germinal center B cells have been identified.4
miR-155 has multiple functions in germinal center B cells, including regulation of class switching, affinity maturation, and lymphocyte motility.4,5 miR-125b promotes the germinal center reaction by inhibiting premature differentiation of germinal center B cells into postgerminal center plasma cells.6 miR-28 is a further miRNA with high expression in germinal center B cells and may function by inhibiting uncontrolled proliferation of these cells.7 Through the work of de Yébenes et al, miR-217 is now established as a further miRNA with a central role in the regulation of the germinal center response. It is shown that miR-217 is upregulated when resting B cells differentiate into germinal center B cells. Enforced expression of miR-217 in B cells in 2 transgenic mouse models and a further approach with downregulation of endogenous miR-217 revealed that miR-217 positively influences the germinal center reaction, leading to increased numbers of germinal center B cells, increased frequencies of class-switched memory B cells, and a higher load of somatic mutations in immunoglobulin V genes in germinal center B cells.1 Further studies led to the identification of >1000 genes that are downregulated by miR-217. Importantly, by ingenuity pathway analysis, a DNA damage response and repair network was revealed as a main target of miR-217 (see figure). Presumably through this way (but perhaps also by additional means), miR-217 contributes to the expression of BCL6, the master regulator of the germinal center B-cell program, and the protection of BCL6 from degradation by DNA damage stress.1
When the miR-217 transgenic mice grew older, many of them developed B-cell lymphomas, typically with a germinal center or postgerminal center origin.1 Thus, miR-217 promotes the development of B-cell lymphomas in mice and can function as an oncogene. Notably, increased expression of miR-217 was also observed in human Burkitt lymphomas and diffuse large B-cell lymphomas in comparison with reactive tissues.1 Although the mechanisms for the upregulated expression of miR-217 in human B-cell lymphomas are still unclear, the oncogenic features of miR-217 in the mouse model indicates that the upregulated expression of miR-217 in human Burkitt and diffuse large B-cell lymphomas contributes to lymphoma pathophysiology.
Overall, the work by de Yébenes et al identified miR-217 as an important positive regulator of the germinal center reaction, but also as a miRNA with oncogenic potential for germinal center B cells. Thereby, this work further substantiates the picture that the germinal center is a dangerous environment for B cells.3,8 Several factors promoting sustained germinal center reactions have been shown to increase the risk for the development of germinal center B cell-derived lymphomas. The main factors for this are most likely the high proliferative activity of the germinal center B cells and their genetic instability due to potential mistakes of the Ig gene remodeling processes of somatic hypermutation and class switching and the dampened DNA damage repair.3,8 miR-217 seems to contribute to all of these risk factors, so that a deregulated activity of this miRNA has oncogenic effects (see figure). It will now be important in future work to clarify in more detail the relevant genes directly regulated by miR-217 and to identify the mechanisms that regulate miR-217 expression and cause its increased expression in human B-cell lymphomas.
Conflict-of-interest disclosure: The author declares no competing financial interests.