In this issue of Blood, Stengel et al advance our knowledge of the genomic landscape of acute lymphoblastic leukemia (ALL) by demonstrating a high frequency of TP53 mutations in specific genetic subtypes.1
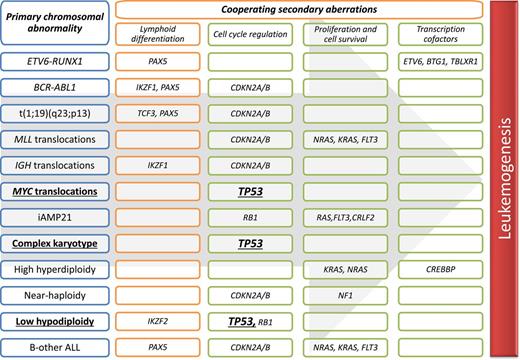
Overview of key cooperating mutations in relation to distinct genetic subtypes of B-cell precursor ALL.
Overview of key cooperating mutations in relation to distinct genetic subtypes of B-cell precursor ALL.
The genomic landscape of ALL is characterized by primary chromosomal abnormalities and a wide range of secondary deletions and mutations, which target key pathways implicated in leukemogenesis. There is a strong correlation between specific primary chromosomal abnormalities and the spectrum of cooperating mutations (see figure). Despite this extensive genetic heterogeneity, TP53 mutations have not previously been reported to occur at a high frequency. This is in contrast to other cancers, especially solid tumors, where TP53 is widely reported as the most frequently altered gene.
Using next-generation sequencing, researchers at the Munich Leukemia Laboratory screened a large cohort of ALL patients for evidence of TP53 mutations. Overall, they observed TP53 mutations in 16% of patients; a frequency much higher than expected. The use of amplicon deep-sequencing enabled the detection of low level clones, which may explain the difference because the mutational load in one-third of patients was <30%. The architecture of TP53 disruption in ALL appears to be similar to other cancers. The vast majority of mutations detected occurred in known mutational hotspots, and there was a strong correlation with deletion of the second allele to generate a so-called double hit.
Although Stengel et al screened 625 patients, the cohort was not typical of ALL as a whole. Children were significantly under-represented, and the cohort included patients with mature B-cell ALL (Burkitt leukemia), which represents a distinct clinical entity. Examination of the data revealed that TP53 mutations were not distributed evenly across ALL subtypes but associated with certain primary abnormalities (see figure). In particular, 3 genetic subtypes had a high frequency of TP53 mutations: low hypodiploidy (92%), MYC translocations (63%), and complex karyotype (23%). Moreover, two-thirds of all TP53 mutations occurred within these 3 genetic subtypes.
The association between low hypodiploidy (30-39 chromosomes) and TP53 mutations has been described before, but because low hypodiploidy is rare, confirmation of this finding was important.2 Chromosome 17 is usually one of the chromosomes lost in low hypodiploidy; therefore, the vast majority of these patients would have a TP53 double hit. Some children with low hypodiploidy were found to harbor germ-line rather than somatic TP53 mutations.2 However, studies of low hypodiploidy in adults, where it is more prevalent, have not revealed a similar association.2,3 Nonetheless, this intriguing observation coupled with the finding, in this study, that low hypodiploid cases have a high TP53 mutational load does question the order with which these abnormalities are acquired. Evidence from relapsed childhood ALL clearly indicates that TP53 mutations and deletions can represent secondary abnormalities that are acquired at relapse.4 However, can they also represent primary events that are capable of inducing low hypodiploidy?
The high frequency of TP53 mutations among patients with a MYC translocation (65%) is a novel observation. TP53 mutations are known to occur in 30% to 40% of patients with Burkitt lymphoma, which is broadly consistent with the frequency of TP53 mutations in other lymphoid malignancies.5 Functional studies have demonstrated that p53 represses c-myc transcription.6 Therefore, loss of p53 function could act to accelerate the oncogenic effect of MYC overexpression. Complex karyotype is not well defined or widely used in ALL classification but does occur in 5% of adult ALL.7 Loss of chromosome arm 17p has been reported in 15% cases with a complex karyotype. Therefore, the observation that 23% of complex karyotypes harbor a TP53 mutation provides the first good evidence of a recurrent lesion within this subtype of ALL.
The frequencies of all 3 TP53-enriched subtypes are known to increase with age.7 Therefore, the higher frequency of TP53 mutations in this cohort, compared with previous studies, is partly explained by the fact that older patients are over-represented. TP53 mutations were much less frequent among those cytogenetic subgroups that characterize pediatric ALL—high hyperdiploidy and ETV6-RUNX1—and therefore they were less common among children as a whole. Nevertheless, Stengel et al report that ∼10% of pediatric ALL (0-19 years) had a TP53 mutation, which is double the frequency reported by the Children’s Oncology Group among high-risk ALL patients.8 Interesting, approximately one-third of TP53 mutations occurred in a minor clone (<30%), and these tended to occur in patients with normal karyotype, BCR-ABL1, MLL translocations, and other abnormalities. In contrast, the TP53 mutational load tended to be higher among patients with low hypodiploidy or a complex karyotype, enforcing the strong link between TP53 mutations and specific cytogenetic subtypes.
Stengel et al report that adult patients with TP53 mutations, especially those harboring a double hit, have an inferior outcome compared with other patients. Given that two-thirds of TP53 patients have low hypodiploidy, MYC translocations, or complex karyotype, all of which are associated with inferior outcome,7 this result is not surprising. However, it should be noted that multivariate analysis did indicate that the effect of TP53 mutations was independent of cytogenetics. As the authors acknowledge, there are limitations with respect to the survival analysis. First, it is restricted to adults; hence, the effect of TP53 among children remains unresolved. TP53 alterations are associated with a poor outcome after first relapse in childhood ALL,4 but whether or not they represent a prognostic biomarker at presentation is unknown. The second issue is treatment heterogeneity. Burkitt leukemia is a distinct subtype of ALL, which is treated alongside Burkitt lymphoma rather than other ALLs. Furthermore, BCR-ABL1 patients (26% of their cohort) are now routinely treated with a tyrosine kinase inhibitor. Further studies based on clinically relevant patient cohorts are required to accurately ascertain the prognostic relevance of TP53 alterations.
This study has identified a clear role for TP53 alterations in specific genetic subtypes of ALL, which are significantly more prevalent in adult compared with childhood ALL and are associated with a very poor prognosis.1,7 The identification of TP53 mutations as a potential therapeutic target could provide alternative treatment strategies for improving the outcome of patients with these subtypes of adult ALL.5
Conflict-of-interest disclosure: The author declares no competing financial interests

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal