In this issue of Blood, Jauréguiberry et al appear to have proven that the hypothesis of early destruction of pitted erythrocytes is correct and propose a method for screening patients for this syndrome.1 Early destruction of pitted erythrocytes has been the leading hypothesis for postartesunate delayed hemolysis since it was first recognized a few years ago.
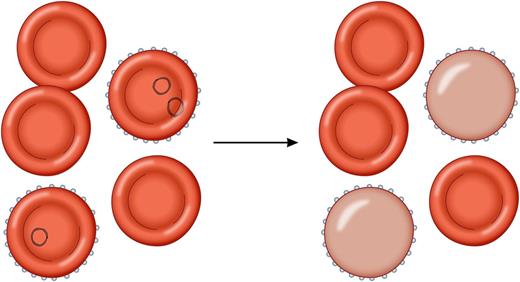
(Left) Malaria-infected erythrocytes. (Right) Parasites killed by artesunate have been removed by the spleen, resulting in a population of once-infected erythrocytes with a considerably decreased life span of about 7 to 21 days. Professional illustration by Patrick Lane, ScEYEnce Studios.
(Left) Malaria-infected erythrocytes. (Right) Parasites killed by artesunate have been removed by the spleen, resulting in a population of once-infected erythrocytes with a considerably decreased life span of about 7 to 21 days. Professional illustration by Patrick Lane, ScEYEnce Studios.
Artesunate is an artemisinin derivative, a class of potent antimalarial medicines. Oral artemisinin compounds in combination therapy and the parenteral counterparts, artesunate and artemether, have become essential tools in the treatment and control of malaria worldwide.2 Two very large multicenter prospective trials demonstrated a survival benefit with artesunate compared with the standard of care regimen for severe malaria (quinine).3,4 In fact, it was noted in those studies that artesunate was well tolerated with essentially no serious drug-related adverse effects. When unexpected brief episodes of hemolytic anemia occurring weeks after treatment of severe malaria with artesunate were reported, it was a difficult puzzle to put together.5 When the hemolytic episodes occurred, they were usually ≥1 week after the symptoms of malaria and parasitemia had resolved, so it did not seem to be a direct effect of the malaria infection. When detected and evaluated, common genetic factors and markers associated with immune-mediated hemolysis were not present. Also, the half-life of artesunate and its active metabolite is extremely short. All would have been completely eliminated from the patient by the time the hemolytic episode started, which seemed to make it unlikely that a direct effect of the medicine was the culprit. In addition, there were also reports of delayed hemolytic anemia after treatment with another artemisinin derivative, artemether, suggesting that this syndrome could occur after treatment with any of the whole class of medicines.6
It had previously been demonstrated that malaria parasites killed by artesunate are removed from erythrocytes by the spleen. These “pitted” erythrocytes (or as referred to by Jauréguiberry et al, once-infected erythrocytes [o-iE]) remain in circulation but with a considerably decreased life span of ∼7 to 21 days.7,8 Also, artemisinins were more likely than quinine to result in the creation of significant numbers of o-iEs. The presence of o-iEs immediately after treatment of severe malaria had not been associated with clinically significant sequelae, but their life cycle seemed to match well with the timeline observed in cases of postartemisinin delayed hemolysis (PADH). This led to the hypothesis for the syndrome of PADH that patients with higher initial parasitemia densities produce higher numbers of o-iEs after treatment with artemisinin drugs. When these erythrocytes with decreased lifespan are cleared en masse, it results in a brief hemolytic episode that does not recur. Jauréguiberry et al demonstrated that the kinetics of the o-iEs correlated with the PADH episodes. In addition to confirming the hypothesis, they described a potentially useful method using flow cytometry for detecting at-risk patients who can be targeted for additional follow-up. Ideally, this method (or an as-yet-undeveloped point-of-care version) will be implemented in the near future at all sites using artemsinins for the treatment of severe malaria. All at-risk patients could be screened for o-iEs immediately after initial treatment to identify impending instances of hemolytic anemia and target those patients for appropriate follow-up.
There are many reasons for anemia among malaria patients including acute hemolysis from the multiplying parasites, dyserythropoesis, excessive nosocomial phlebotomy, transfusion reactions, and medication side effects. Most patients who have survived an episode of severe malaria will be anemic, with a slow recovery to baseline. It can be difficult to distinguish PADH when superimposed on that abnormal background. A close examination of the case reports of purported PADH revealed several cases that do not fit this pattern, should not have been included, and are artificially inflating the incidence estimates.5 There are no additional patterns of anemia for PADH. Case reports of patients demonstrating other patterns of anemia are most often experiencing the usual response to malaria infection and the complex interplay of the other malaria-related causes of anemia and not PADH.
Surveillance efforts should focus on detecting a clinically significant loss of erythrocytes from the recovering patient’s already abnormally low baseline. A lax case definition will result in overestimates, such as the 20% to 25% incidence that has been suggested. A recent article using a case definition of any decrease in hemoglobin between days 7 and 14 after treatment plus either a low haptoglobin or high serum lactate dehydrogenase (LDH) reported an incidence of 7%.9 However the decrement in hemoglobin was <1 g/dL in all but 1 patient. Jauréguiberry et al suggest a 10% decrement in hemoglobin associated with haptoglobin <0.1 g/L and either an increase in LDH to >390 IL/L or a 10% rise >7 days after treatment initiation with artesunate. Although a 10% drop in hemoglobin in an already anemic person will also result in persons with relatively minor fluctuations in their hemoglobin level being included as cases, this approach improves specificity and should be adopted as the case definition with 1 important addition. PADH is a nonrecurring hemolytic episode. Inclusion of this qualifier will further improve the specificity of the case definition.
It is important to note that the study by Jauréguiberry et al supports the clinical experience on the overall safety of artesunate. PADH seems to be uncommon, potentially predictable, and does not seem to be a direct effect of artemisinin derivative drugs. These erythrocytes were already sentenced to death when they were infected by malaria parasites, and artesunate allowed these infected erythrocytes a temporary stay of execution.
Conflict-of-interest disclosure: P.M.A. is the principal investigator for the Investigational New Drug Protocol: Intravenous Artesunate for Treatment of Severe Malaria in the United States, at the Centers for Disease Control and Prevention.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal