In this issue of Blood, Kagoya et al provide evidence for an important role for factors secreted by leukemia cells in damaging and suppressing normal hematopoiesis.1
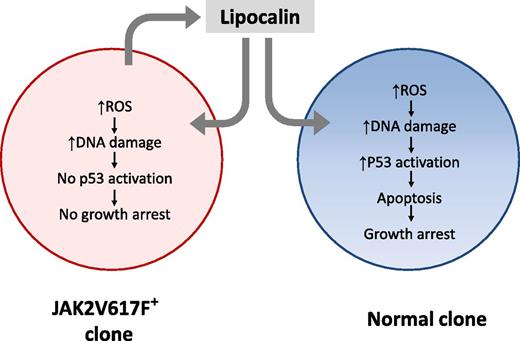
Secretion of Lcn2 by JAK2V617F+ MPN cells induces ROS and DNA damage in coexisting JAK2V617F− normal cells, leading to p53 activation, cell-cycle arrest, and apoptosis. Lcn2 also induces ROS and DNA damage in JAK2V617F+ neoplastic cells, but an impaired p53 response in these cells may prevent growth impairment, providing them with a selective growth advantage. Lcn2-induced DNA damage could potentially contribute to acute leukemic transformation of JAK2V617F+ and JAK2V617F− cells from MPN patients to AML.
Secretion of Lcn2 by JAK2V617F+ MPN cells induces ROS and DNA damage in coexisting JAK2V617F− normal cells, leading to p53 activation, cell-cycle arrest, and apoptosis. Lcn2 also induces ROS and DNA damage in JAK2V617F+ neoplastic cells, but an impaired p53 response in these cells may prevent growth impairment, providing them with a selective growth advantage. Lcn2-induced DNA damage could potentially contribute to acute leukemic transformation of JAK2V617F+ and JAK2V617F− cells from MPN patients to AML.
Recently, much attention has been paid to the role of leukemia-induced changes in the bone marrow microenvironment in selectively promoting growth of leukemia while suppressing the growth of normal stem cell growth. Suppression of normal hematopoiesis is a commonly observed feature of leukemia, but the underlying mechanisms are not well understood. Establishment and progression of leukemia in vivo require a competitive advantage of leukemic over coexisting normal stem cells in the bone marrow. Although this could be attributed to intrinsic proliferative and survival advantages of leukemic cells resulting from a variety of genetic and epigenetic alterations, differential microenvironmental regulation of leukemic and normal stem cells appears to be an important factor contributing to the establishment of leukemia, especially at early stages of development where normal hematopoiesis may be dominant.2
Studies in chronic myelogenous leukemia (CML), myeloproliferative neoplasm (MPN), and acute myeloid leukemia (AML) have shown the importance of leukemia-induced changes in the microenvironment in promoting disease progression. In CML, it has been shown that in addition to intrinsic BCR-ABL–induced alterations in stem cell growth, leukemia cells induce alterations in chemokine and cytokine expression in the bone marrow microenvironment that contribute to enhanced growth of CML stem cells and suppression of normal stem cells, and provide a selective growth advantage to CML stem cells.3 Interestingly, abnormalities in microenvironmental regulation are improved but not completely corrected by tyrosine kinase inhibitor treatment. BCR-ABL–expressing cells have been shown to induce a remodeling of the niche to their advantage, accompanied by a secondary compromise of their normal cell competitors.4 Recent studies have identified leukemia-induced disruption of sympathetic nerve fibers within the bone marrow of MPN patients and mice expressing the human JAK2V617F mutation, and in mixed-lineage leukemia gene–induced AML models.5,6 Sympathetic nerve damage results in alterations in mesenchymal cells that promote growth of leukemic stem cells at the expense of normal stem cells.
The studies of Kagoya et al extend our understanding of leukemia-induced alterations in normal and leukemic hematopoiesis and its contribution to leukemogenesis. Using a murine model of JAK2V617F+ MPN, they show that malignant cells not only demonstrated increased reactive oxygen species (ROS) themselves, but also induced elevated ROS and paracrine DNA damage in neighboring normal cells. A screen of candidate factors based on analysis of gene expression and short hairpin RNA–mediated knockdown identified overexpression of lipocalin-2 (Lcn2) in JAK2V617F+ cells as being a significant contributor to paracrine DNA damage. Lcn2 expression in MPN was Janus kinase/signal transducer and activator of transcription (JAK/STAT) pathway dependent. In normal hematopoietic cells, Lcn2 treatment resulted in elevated ROS levels, leading to p53 pathway activation, increased apoptosis, and decreased cellular proliferation (see figure). In contrast, JAK2V617F+ cells were not susceptible to Lcn2-induced growth suppression despite elevation of ROS and induction of DNA damage, possibly related to attenuated p53 activation in these cells. These findings suggest that Lcn2, in addition to providing JAK2V617F+ cells with a relative growth advantage by suppressing growth of coexisting normal hematopoietic cells, could also accelerate leukemogenesis through induction of DNA damage.
Previous studies have also identified Lcn2 as being essential to BCR-ABL–induced leukemogenesis.7 Reduction of Lcn2 expression by antisense or small interfering RNA approaches strongly reduced the growth of BCR-ABL–expressing cell lines or primary cells in mouse models in vivo. Their studies suggested that Lcn2 contributes to leukemogenesis via dual mechanisms of induction of apoptosis in normal hematopoietic cells and enhancement of tissue invasion by leukemia cells. These results in a CML model are consistent with those of the current study in MPN, and suggest that Lcn2 expression could represent a conserved mechanism that promotes progression of myeloid malignancies. Additional studies evaluating the effect of Lcn2 deletion using genetic mouse models on leukemic and normal hematopoiesis are required to more definitively determine its contribution to leukemia progression.
The studies of Kagoya et al also have implications for the evolution of MPN to AML, suggesting a possible contribution of Lcn2-induced ROS and DNA damage to acquisition of mutations in both JAK2V617F+ and JAK2V617F− cells. It is recognized that AML arising from JAK2V617F+ MPN is often JAK2V617F−. This has been explained by existence of JAK2V617F− preleukemic populations that may bear mutations in other genes, such as TET2, which could confer clonal preservation or expansion.8 The current studies showing that JAK2V617F− normal clones accumulated DNA damage to the same extent as JAK2V617F+ clones in the MPN mouse model are intriguing, but further evaluation is needed to determine whether Lcn2-induced induction of ROS and DNA damage indeed contributes to leukemia evolution in MPN models. Confirmation of such a role may offer additional strategies to alter the progression of MPN and enhance response to treatment.
Conflict-of-interest disclosure: The author declares no competing financial interests.