Key Points
Moderate-dose cyclophosphamide is associated with an unacceptable rate of toxicity in SAA, as in high-dose cyclophosphamide.
Moderate-dose cyclophosphamide is an active regimen but is associated with a low response and does not prevent relapse or clonal evolution.
Abstract
First-line therapy of severe aplastic anemia (SAA) with high-dose cyclophosphamide causes toxicity and increased short-term mortality. We investigated cyclophosphamide at a lower, more moderate dose in combination with aggressive supportive care to determine whether severe infections might be avoided and hematologic outcomes defined for this regimen. From 2010 to 2012, 22 patients received cyclophosphamide at 120 mg/kg plus cyclosporine and antibacterial, antiviral, and antifungal prophylaxis. Toxicity was considerable, mainly due to prolonged absolute neutropenia, which occurred regardless of pretherapy blood counts, and persisted an average of 2 months. Granulocyte transfusions for uncontrolled infection were required in 5 patients, confirmed fungal infections were documented in 6, and 9 patients died. Nine patients (41%) responded at 6 months. After a median follow-up of 2.2 years, relapse occurred in 2 patients, and cytogenetic abnormalities (including monosomy 7) were observed in 4 patients. Although cyclophosphamide has activity in SAA, its toxicity is not justified when far less dangerous alternatives are available. This trial was registered at www.clinicaltrials.gov as #NCT01193283.
Introduction
Severe aplastic anemia (SAA) is a life-threatening hematologic disease characterized by pancytopenia and hypocellular bone marrow. Because most patients lack a histocompatible donor, the majority are treated with standard immunosuppressive therapy (IST) with horse antithymocyte globulin (h-ATG) plus cyclosporine (CsA).1,2 Standard IST is effective and has markedly improved survival in SAA. Nevertheless, current limitations of IST are lack of hematologic response (in ∼33%), incomplete responses, relapses (in ∼33%), and clonal evolution to myelodysplastic syndrome and acute myeloid leukemia (in ∼15%).2-6 Efforts to improve IST in treatment-naïve patients by the addition of mycophenolate mofetil and sirolimus to standard h-ATG/CsA or use of more lymphocytotoxic agents such as rabbit ATG or alemtuzumab have failed to produce better outcomes.1,2,7-9
Cyclophosphamide (Cy) has been proposed as an alternative to h-ATG/CsA.10 A single-institution phase 2 study suggested that high-dose Cy (200 mg/kg) yielded results similar to those observed for h-ATG/CsA but with fewer relapses and clonal evolutions.11 However, in a randomized study conducted at the National Institutes of Health that directly compared high-dose Cy and h-ATG/CsA in treatment-naïve patients, excess toxicity and death from invasive fungal infections were observed in the Cy arm, which led to early termination of the protocol.12 A recently published Chinese study that used a lower, more moderate dose of Cy (120 mg/kg total) plus CsA reported results similar to those in patients concurrently treated with ATG, and there was a more abbreviated duration of neutropenia and less toxicity than with the 200 mg/kg Cy regimen.13 Because the marked improvement in survival in SAA among those who did not respond to IST coincides with the introduction of improved antifungal drugs,14 we considered it reasonable to investigate moderate-dose Cy as definitive therapy in SAA. Here we report outcomes in treatment-naïve patients who received moderate-dose Cy as first-line therapy.
Methods
Patients
Twenty-two consecutive patients were enrolled in a treatment protocol that investigated moderate-dose Cy/CsA. All patients (or their legal guardians) signed informed consent according to protocols approved by the Institutional Review Board of the National, Heart, Lung, and Blood Institute, in accordance with the Declaration of Helsinki. All patients were treated at the Warren G. Magnuson Clinical Center in Bethesda, MD.
Eligibility and end points
All patients 2 years old (and weighing 12 kg) or older were eligible to participate. The main objective was to assess the safety and efficacy of Cy at 120 mg/kg + low-dose CsA. The primary hematologic end point was response, defined as no longer meeting criteria for SAA at 6 months. The complete inclusion, exclusion, and secondary end points are included in the supplemental Data available on the Blood Web site.
IST
Cy was administered at 30 mg/kg by intravenous infusion over 60 minutes once daily for 4 days (total dose of 120 mg/kg). CsA dosing was adjusted to obtain a therapeutic trough goal level between 100 and 200 μg/L. CsA was initiated orally and was administered for 6 months and then discontinued. Details of granulocyte colony-stimulating factor, valacyclovir, ciprofloxacin, trimethoprim-sulfamethoxazole, and voriconazole (trough goal between 1 and 5.5 mg/L) dosing are included in the supplemental Data.
Statistical methods
The study was designed to show an increase in complete response (CR) rate >30%, which in our experience is a surrogate for fewer late events. We hypothesized that the actual CR probability using this treatment would reach 30% or more and that a CR probability of 10% or less would warrant terminating the protocol. We determined the sample size using the 2-stage minimax design of Simon. At α = .05 significance level and β = .80 power, 15 patients were accrued in the first stage, and the null hypothesis was accepted if no more than 1 participant achieved a CR. If 2 or more patients attained a CR within 6 months at the first stage, an additional 10 patients would be accrued (n = 25 total). Additional details of statistical methods are available in the supplemental Data.
Results and discussion
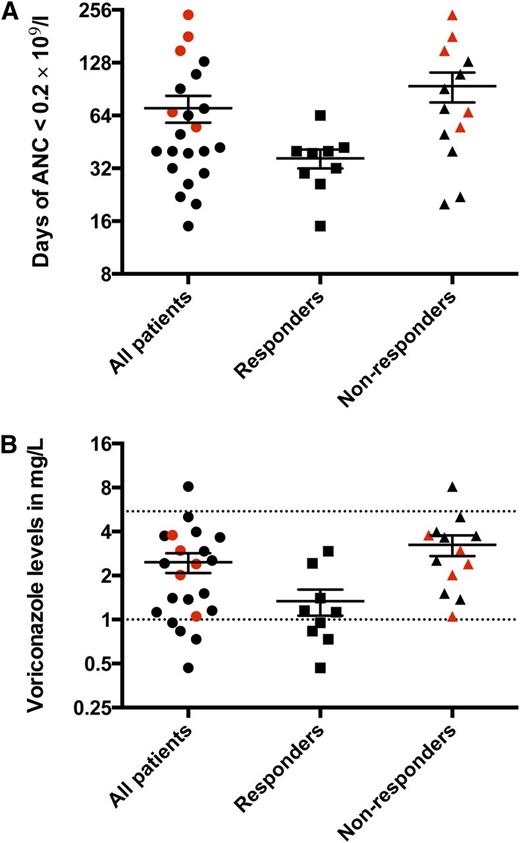
In total, 22 patients received Cy/CsA from September 2010 to February 2012. In March 2012, the Data Safety Monitoring Board recommended termination of accrual because of unacceptable toxicity as well as the occurrence of clonal evolution and deaths. The results for the 22 accrued patients are reported here. Patient characteristics are shown in supplemental Table 1. There were no paroxysmal nocturnal hemoglobinuria-related hemolytic or thrombotic complications in this trial. The median follow-up was 2.2 years (range, 0.4 to 3.4 years). An absolute granulocyte count of 0 × 109/L was universal after Cy, irrespective of baseline neutrophil levels. The average duration of severe neutropenia (<0.2 × 109/L) was approximately 2 months (Figure 1A). During this period, infectious events were serious and frequent, despite aggressive antibacterial, antiviral, and antifungal prophylaxis, with particular attention on targeting therapeutic voriconazole levels (Figure 1B and supplemental Table 2). Granulocyte transfusions were required in 5 patients for uncontrolled infections. Confirmed filamentous fungal infections were documented in 6 patients (supplemental Table 2). The average duration of initial inpatient hospitalization was 47 days (range, 23 to 127 days), and the average number of hospitalizations was 2.1 (range, 1 to 9 hospitalizations) within the first 6 months after Cy.
Duration of very severe neutropenia (<0.2 × 109/L) after Cy and voriconazole levels. (A) The average duration of very severe neutropenia was 70 days (range, 15 to 240 days) for the entire cohort, whereas for responders it was 36 days (range, 15 to 64 days), and for nonresponders, it was 94 days (range, 20 to 240 days). (B) The average voriconazole level for the entire cohort was 2.4 mg/L (range, 0.47 to 8.130 mg/L), among responders, it was 1.3 mg/L (range, 0.47 to 2.9 mg/L), and among nonresponders, it was 3.2 mg/L (range, 1.1 to 8.1 mg/L). Each data point represents the average voriconazole level for each patient throughout the duration of very severe neutropenia. The target voriconazole level was 1 to 5.5 mg/L (dotted lines). The patients who required granulocyte transfusions for uncontrolled infections despite maximum antimicrobial support are depicted in red. ANC, absolute neutrophil count.
Duration of very severe neutropenia (<0.2 × 109/L) after Cy and voriconazole levels. (A) The average duration of very severe neutropenia was 70 days (range, 15 to 240 days) for the entire cohort, whereas for responders it was 36 days (range, 15 to 64 days), and for nonresponders, it was 94 days (range, 20 to 240 days). (B) The average voriconazole level for the entire cohort was 2.4 mg/L (range, 0.47 to 8.130 mg/L), among responders, it was 1.3 mg/L (range, 0.47 to 2.9 mg/L), and among nonresponders, it was 3.2 mg/L (range, 1.1 to 8.1 mg/L). Each data point represents the average voriconazole level for each patient throughout the duration of very severe neutropenia. The target voriconazole level was 1 to 5.5 mg/L (dotted lines). The patients who required granulocyte transfusions for uncontrolled infections despite maximum antimicrobial support are depicted in red. ANC, absolute neutrophil count.
In a companion protocol (also terminated), 1 patient received Cy at 60 mg/kg along with fludarabine 125 mg/m2 (NCT01187017) for refractory SAA. Again, toxicities were grave, with prolonged neutropenia and fungal pulmonary infection. Thus, 2 patients with adequate pretreatment neutrophil counts (>1 × 109/L) prior to a Cy-based regimen never recovered granulocytes, leading to extremely prolonged hospitalization and ultimately death after unsuccessful salvage hematopoietic stem cell transplantation (HSCT).
Overall, 9 patients (41%; 95% CI, 20% to 62%) responded to moderate-dose Cy at 6 months: 4 CRs (18%) and 5 partial responses (23%). There were no CRs at 3 months. Among nonresponders at 6 months, 5 later underwent HSCT, 4 from a sibling donor and 1 from an unrelated donor (one patient received h-ATG/CsA prior to undergoing HSCT). The conditioning regimen for the sibling HSCT was based on fludarabine/Cy/h-ATG. One patient died between 3 and 6 months after initial immunosuppression. Of the 7 remaining unresponsive patients, 5 underwent a second course of immunosuppression with standard h-ATG plus CsA (1 responded), and 2 died after the 6-month landmark from infectious complications.
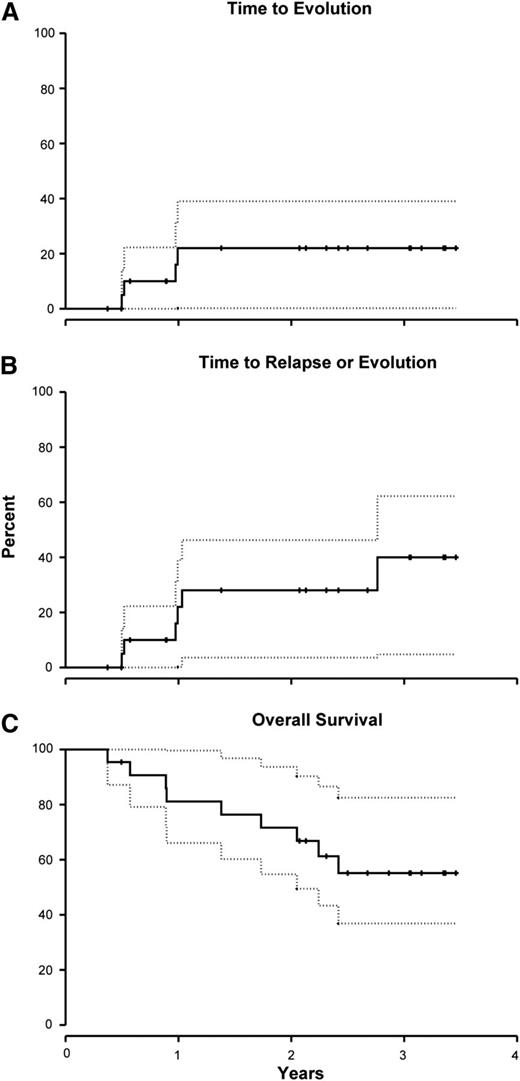
The cumulative incidence of clonal evolution at 1 year was 22% (95% CI, 0% to 39%; Figure 2A). The cytogenetic abnormalities among the 4 evolutions were trisomy 15 (1 patient), del20q (1), del7q (1), and monosomy 7 (1). The 2 patients with chromosome 7 abnormalities were nonresponders, whereas the other 2 achieved a partial response. All but the patient with trisomy 15 died. Two additional patients relapsed, bringing the incidence of late events (relapse or clonal evolution) to 28% (95% CI, 4% to 46%; Figure 2B). To date, all but 1 of the responding patients are alive. The 2-year overall survival for this cohort was 72% (95% CI, 55% to 94%) (Figure 2C). The causes of death were pulmonary mucormycosis in 1 patient, necrotizing fasciitis (1), septicemia (1), evolution to leukemia (1), unknown (1), and after HSCT (4) (3 patients underwent HSCTs for unresponsive disease and 1 for clonal evolution).
Clonal evolution, late events (relapse + clonal evolution), and survival rates following Cy + CsA. (A) The cumulative incidence of clonal evolution at 1 year was 22% (95% CI, 0% to 39%). (B) An additional 2 patients relapsed, bringing the total incidence of late events (relapse + clonal evolution) to 28% (95% CI, 4% to 46%) at 2 years. (C) The 2-year survival for this cohort was 72% (95% CI, 55% to 94%). Day 0 in this survival curve is the first day of Cy. Dotted lines represent 95% confidence intervals.
Clonal evolution, late events (relapse + clonal evolution), and survival rates following Cy + CsA. (A) The cumulative incidence of clonal evolution at 1 year was 22% (95% CI, 0% to 39%). (B) An additional 2 patients relapsed, bringing the total incidence of late events (relapse + clonal evolution) to 28% (95% CI, 4% to 46%) at 2 years. (C) The 2-year survival for this cohort was 72% (95% CI, 55% to 94%). Day 0 in this survival curve is the first day of Cy. Dotted lines represent 95% confidence intervals.
We show that the toxicity with moderate-dose Cy is substantial and similar to that observed with the higher Cy dose of 200 mg/kg. These results were disappointing because administration of 60% of the total Cy dose and institution of modern aggressive supportive and antimicrobial therapy did not avoid prolonged neutropenia and its anticipated dire consequences. Initial hospitalization was extended much beyond in-hospital stays with h-ATG–based therapies (for which patients are usually discharged in 1 to 2 weeks).15 We were not able to reproduce the Chinese experience, in which duration of neutropenia was described as abbreviated to 1 month with a low rate of infectious complications.13 Unknown ethnic differences in Cy pharmacokinetics could contribute to the different toxicity outcomes.16,17 The occurrence of late events of hematologic relapse and clonal evolution were also unsatisfactory (Figure 2B). In this cohort, these events occurred early, which differs from the experience with ATG therapies in which late events usually occur after the first year from immunosuppression.2,15
We consider the prophylactic regimen used in this trial to be optimal. All patients received antibacterial, antiviral, and broad-spectrum antifungal prophylaxis with voriconazole, which has activity against Aspergillus.14,18 The target voriconazole dosing ensured that breakthrough fungal infections would not be consequent to underdosing, but this strategy did not appear effective in curtailing many lethal infections in our cohort.19,20 The routine use of a quinolone prophylaxis was also not efficacious. Many infections were secondary to gram-positive organisms, likely selection pressure from routine use of ciprofloxacin (supplemental Table 2).21 Fungal infections resistant to voriconazole were also observed at a higher frequency than in previous trials at our institution, which does not routinely use voriconazole. Thus, we believe that maximal prophylactic therapy, as used in this study, failed to prevent infectious morbidity and mortality. In recent surveys and formal policy recommendations, regimens based on Cy are either not included or are used only in a single center,22,23 reflecting appropriate concern with the toxicity associated with this drug.
Thus, we conclude that moderate Cy/CsA, although it is capable of producing meaningful hematologic responses in some patients, results in significant infectious toxicity, with long and frequent hospitalizations despite maximum prophylactic and intensive supportive care. Because of the high toxicity of moderate Cy/CsA, response rates of 40% to 50%, and no benefit from a relapse and clonal evolution standpoint, we cannot justify investigating this regimen further. Although Cy has activity in SAA, its toxicity is not justified when far less toxic alternatives such as h-ATG are available.
The online version of this article contains a data supplement.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the Intramural Research Program of the National Heart, Lung, and Blood Institute of the National Institutes of Health.
Authorship
Contribution: Phillip S. was the principal investigator for the protocols, and he conceptualized and conducted the clinical trials, analyzed the data, interpreted the results, and drafted the manuscript. O.R. and B.W. performed the patient screening and data collection and attended to all patients’ needs; Priscila S. and M.D. attended to regulatory protocol requirements including data collection; C.O.W. was involved in the conceptualization, statistics, and writing of the protocols and performed the statistical analysis for the manuscript; and N.S.Y. was involved in conceptualizing, implementing, and writing the protocols and conducting the protocols, interim discussions, data analysis, interpretation of results, and writing of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Corresponding author: Phillip Scheinberg, Hospital São José, Rua Martiniano de Carvalho, 951, Bela Vista, São Paulo, Brazil 01321-001; e-mail: scheinbp@gmail.com.