In this issue of Blood, Krevvata et al show that osteoblasts duel with acute leukemia cells, each having adverse effects on the other.1
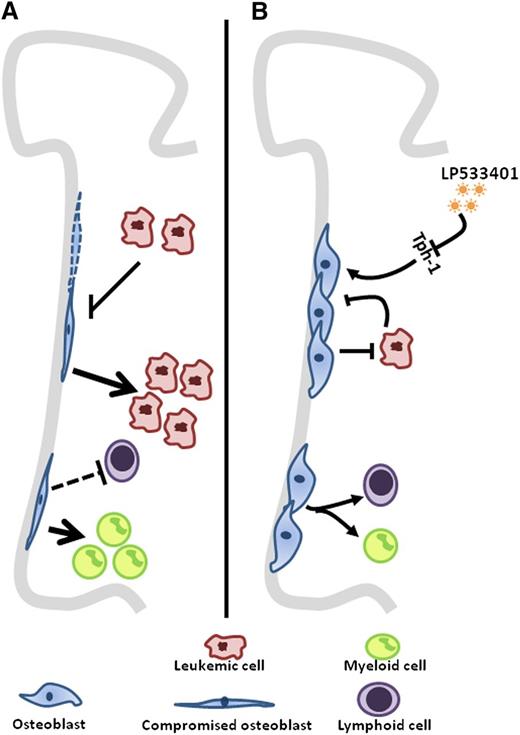
(A) A decrease in osteoblast numbers in mostly untreated AML/MDS patients is shown. In a DTAosb animal model where osteoblasts were deleted, there was a threefold increase in disease burden in addition to an alteration of lineage determination. (B) Conversely, increasing osteoblast numbers through the use of a Tph-1 inhibitor, LP533401, resumed normal hematopoiesis and resulted in a decrease of disease burden translated by a prolonged survival of leukemic mice.
(A) A decrease in osteoblast numbers in mostly untreated AML/MDS patients is shown. In a DTAosb animal model where osteoblasts were deleted, there was a threefold increase in disease burden in addition to an alteration of lineage determination. (B) Conversely, increasing osteoblast numbers through the use of a Tph-1 inhibitor, LP533401, resumed normal hematopoiesis and resulted in a decrease of disease burden translated by a prolonged survival of leukemic mice.
The notion that cancer is a disease of tissues rather than that of a single rogue cell type has increasingly gained ground, particularly in the setting of hematologic malignancies. Although a single cell type generally exerts the pathologic consequences of malignancy, there is accumulating evidence that other cell types can contribute to the initiation, maintenance, and progression of the neoplasia. The report by Krevvata et al adds evidence that osteoblast loss is a necessary component of acute leukemic progression.1 Reducing that loss mitigated the leukemia.
Tumor stroma has long been regarded as a coconspirator in the malignant process. Cancer-associated fibroblasts (CAFs) are not the same as fibroblasts from surrounding normal tissue in solid tumors like those of the breast, colon, or lung. The CAFs have been implicated in inducing neoangiogenesis, extracellular matrix reorganization, metabolic context, and growth factor milieu to enhance cancer progression.2 These mesenchymal cells are not alone in the tumor-supporting cast because hematopoietic populations resident in tumors have become of central interest in tumor progression. Among these, macrophages are the most abundant and provide cytokines such as epidermal growth factor to enhance malignant cell growth and vascular endothelial growth factor and Tie2 to promote neoangiogenesis.3 They both respond to the presence of cancer cells and feedback to the cancer cells. This includes metabolites because cancer-cell lactate production induces M2 polarization of macrophages. These M2 cells have high levels of arginase-1 known to dampen lymphocyte activity, and perhaps also enhance polyamine synthesis needed by tumor cells for their propagation.4 Thus solid tumors have co-opted cells in their environment such that these stromal components enable cancer cell growth.
A similar paradigm has been shown in hematopoietic neoplasms where, for example, myeloproliferative neoplasm (MPN) cells induced increased osteoblastic cells and marked gene expression changes in mesenchymal stromal cells. Increased transforming growth factor-β and decreased CXCL12 and kit ligand (among other changes) compromised stromal cell support of normal hematopoietic cells in vitro, but not malignant ones.5 Tumor cells were preferentially supported over their normal counterparts in the coculture system.
However, the co-evolution of neoplastic cells and their stromal components also has a more hostile face in hematologic malignancies. Some cells of the bone marrow microenvironment appear to drop out in the presence of malignancy. Krevvata et al showed that this is true of osteoblastic cells in the setting of acute myeloid leukemia (AML) or myelodysplasia (MDS) among patients, and in mice models of acute lymphoblastic or myeloid leukemia (see figure). Patients had lost almost half of their osteoblasts even before therapy. This is in agreement with a previous report demonstrating functional inhibition of osteoblasts in a murine AML model.6 In addition, 2 laboratories recently showed that sympathetic neurons are adversely affected by the presence of JAK2-induced MPN or MLL-AF9–induced AML cells, respectively.7,8 In each case, modifying the number of these microenvironmental cells affected the growth kinetics of the neoplasm. Reducing nestin+ mesenchymal stem cells (MSCs)7 or inhibiting adrenergic receptors in sympathetic neurons8 (β3 for MPN, β2 for AML) increased the aggressiveness of the neoplastic disease in vivo. The converse was seen when nestin+ MSCs were protected through the use of adrenergic agonists, which restored the sympathetic control of these cells. Therefore, it seems that some myeloid neoplasms (and perhaps acute lymphoblastic leukemic cells as well) reduce niche elements that do not support them. The reduction in those niche components appears to be a key contributor to in vivo disease kinetics and a possible cause for the suppression of normal hematopoeisis.1 Parathyroid hormone injections to mitigate the loss of niche osteolineage cells in studies of BCR/ABL disease9 or adrenergic agonists in MPN7 delayed disease progression, respectively. The potency of any of these interventions is unlikely to be sufficient to control disease, but these early studies indicate that a focused effort on niche-targeted therapeutics may be justified. Combining niche support with conventional therapies targeting the neoplastic cell may serve to suppress the malignancy while restoring normal hematopoeisis, thus improving a patient’s chances in this duel with disease.
Conflict-of-interest disclosure: The authors declare no competing financial interests.