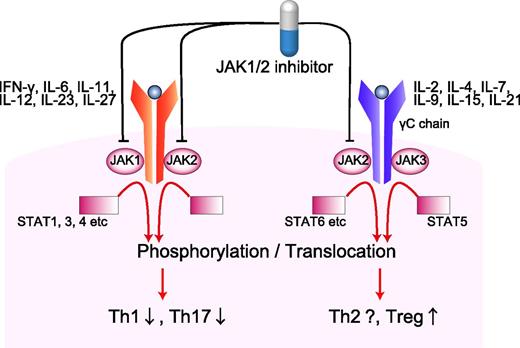
In this issue of Blood, Spoerl and colleagues demonstrate a promising strategy to control graft-versus-host disease (GVHD) after allogeneic hematopoietic stem cell transplantation (HSCT) by using a Janus kinase (JAK) inhibitor in both mice and humans.1
Proinflammatory cytokines, such as IFN-γ, IL-6, and IL-12, ignite serial phosphorylation of JAK1/2, cytokine-receptor chains, and STAT1/3/4. Phosphorylated STATs form heterodimers or homodimers translocate to nucleus, leading to the transcriptions of Th1- or Th17-related genes. On the other hand, γC subunits of IL-2, IL-4, IL-7, IL-15, and IL-21 receptors are associated with JAK3 and STAT5 signaling. JAK1/2 selective inhibition spared the IL-2–JAK3–STAT5 signal and therefore may spare Tregs. Th, T helper.
Proinflammatory cytokines, such as IFN-γ, IL-6, and IL-12, ignite serial phosphorylation of JAK1/2, cytokine-receptor chains, and STAT1/3/4. Phosphorylated STATs form heterodimers or homodimers translocate to nucleus, leading to the transcriptions of Th1- or Th17-related genes. On the other hand, γC subunits of IL-2, IL-4, IL-7, IL-15, and IL-21 receptors are associated with JAK3 and STAT5 signaling. JAK1/2 selective inhibition spared the IL-2–JAK3–STAT5 signal and therefore may spare Tregs. Th, T helper.
GVHD is mediated by cytotoxic T-cell effectors and dysregulated inflammatory cytokines and remains a major cause of morbidity and mortality after allogeneic HSCT. Corticosteroids are the mainstay of GVHD therapy but no standard salvage therapy has been established for corticosteroid refractory GVHD. Spoerl and colleagues report that JAK1/2 inhibitor ruxolitinib is effective to control GVHD.1
JAK inhibitors block JAK–signal transducer and activator of transcriptional factor (STAT) signaling and are approved for the treatment of myelofibrosis.2,3 JAK inhibitors relieve symptoms related to an excess of proinflammatory cytokines in patients with myelofibrosis, regardless of JAK2 mutational status. In light of the inflammatory nature of GVHD, would JAK inhibitors regulate GVHD? JAK-STAT pathways are also expressed in T cells and therefore also play an important role in regulation of the immune system. T cells are activated in a 3-step process by receiving 3 sequential signals from antigen-presenting cells (APCs). Signal 1 is mediated through T-cell receptor binding to major histocompatibility complex molecules. Signal 2 is the costimulatory signal. Signal 3 is the polarizing signal from APCs, such as cytokines that determine the fate of T-cell differentiation into effector cells. In T cells, JAK1/2 relays the signaling function of many inflammatory cytokines with relevance for GVHD, including interferon-γ (IFN-γ), interleukin-2 (IL-2), IL-6, IL-12, and IL-23 (see figure).
Spoerl and colleagues show that inhibition of JAK1/2 signaling by ruxolitinib reduces GVHD in a mouse model by inhibiting donor T-cell expansion and inflammatory cytokine production, and favoring regulatory T-cell (Treg) differentiation.1 Based on these observations in mice, the authors then evaluated whether their findings can be translated into the clinic. Six patients with steroid refractory GVHD that had been heavily pretreated were treated with ruxolitinib. All patients are reported to have responded to ruxolitinib with improved GVHD grades and corticosteroid-sparing effects. Despite the small number of patients in this single-center study, the quick and profound responses to ruxolitinib are very impressive.
This study will pave the way to developing a novel therapeutic strategy of GVHD. However, there are many questions remaining to be addressed. First, it has been shown that ruxolitinib also potently impairs APC functions.4 It remains unclear whether reduction of GVHD is chiefly mediated by T-cell suppression, Treg generation, or APC inhibition. Furthermore, mechanisms of GVHD suppression in patients are unclear; clinical study focuses on treatment of steroid refractory GVHD, whereas the mouse study evaluates prevention of GVHD without steroids. Second, accumulating evidence indicates that ruxolitinib exerts substantial immunosuppressive activity in patients with myelofibrosis, with increases in hepatitis B virus activation and opportunistic infections.5 JAK-STAT pathways are also essential for cytokine-mediated hematopoiesis; thrombocytopenia and anemia are the major adverse effects of ruxolitinib in myelofibrosis. Thus, use of ruxolitinib after allogeneic HSCT can be a double-edged sword. In this study, the lower ruxolitinib dose of 10 to 20 mg per day was used compared with a standard dose of 30 to 40 mg per day used in myelofibrosis trials.2,3 Further studies to determine optimal dose and duration of the therapy are warranted to allow better GVHD control, while avoiding profound immunosuppression and cytopenias in allogeneic HSCT patients who have a fragile hematopoiesis and immune system. Finally, ruxolitinib blocks multiple cytokine signaling and affects multiple processes of immunity. However, compared with ruxolitinib, corticosteroids appear to exert much broader effects on multiple cell lineages, while calcineurin inhibitors pinpoint a target downstream of IL-2 signaling in T cells. Better understanding of the difference in mechanisms of action of these 3 classes of immunosuppressants should lead to optimal utilization of JAK inhibitors in the prophylaxis and treatment of GVHD. JAK1/2 selective inhibition spares the IL-2–JAK3–STAT5 signal that is critical for proliferation and survival of Tregs (see figure), but this beneficial effect may be abrogated when combined with other immunosuppressants.6 Thus, this study suggests that JAK inhibitors may have activity in GVHD and future studies will have to address the efficacy of this approach, when used alone and in combination with other classes of immunosuppressants.
Conflict-of-interest disclosure: The author declares no competing financial interests.