In this issue of Blood, Chen et al show that heme is released during erythrocyte hemolysis in sickle cell disease, activating the innate immune response and triggering the release of neutrophil extracellular traps (NETs) to promote lung injury.1
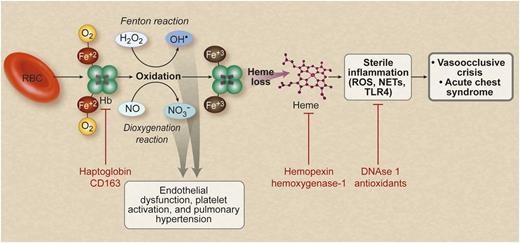
Hemolysis releases erythroid DAMP molecules to drive vascular injury and sterile inflammation, which contribute to the pathogenesis of sickle cell disease. Hemolysis releases cell free hemoglobin (Hb), which is normally scavenged by haptoglobin and CD163. Free hemoglobin reacts with and scavenges NO via the dioxygenation reaction and also reacts with hydrogen peroxide to generate hydroxyl radicals via the Fenton reaction. This process leads to endothelial dysfunction and pathological vascular remodeling. Oxidized hemoglobin releases free heme, which can trigger a sterile inflammatory reaction involving TLR4 activation, and stimulates neutrophils to release NETs. These inflammatory events are proposed to cause vasoocclusion and acute chest syndrome in sickle cell disease. There are several potential therapies using the indicated agents (shown in red text) that target multiple stages of this proposed pathophysiological pathway. RBC, red blood cell. Professional illustration by Debra T. Dartez.
Hemolysis releases erythroid DAMP molecules to drive vascular injury and sterile inflammation, which contribute to the pathogenesis of sickle cell disease. Hemolysis releases cell free hemoglobin (Hb), which is normally scavenged by haptoglobin and CD163. Free hemoglobin reacts with and scavenges NO via the dioxygenation reaction and also reacts with hydrogen peroxide to generate hydroxyl radicals via the Fenton reaction. This process leads to endothelial dysfunction and pathological vascular remodeling. Oxidized hemoglobin releases free heme, which can trigger a sterile inflammatory reaction involving TLR4 activation, and stimulates neutrophils to release NETs. These inflammatory events are proposed to cause vasoocclusion and acute chest syndrome in sickle cell disease. There are several potential therapies using the indicated agents (shown in red text) that target multiple stages of this proposed pathophysiological pathway. RBC, red blood cell. Professional illustration by Debra T. Dartez.
Sickle cell disease is characterized by intraerythrocytic hemoglobin S polymerization that leads to vasoocclusive events and chronic hemolytic anemia. Hemolysis, although traditionally considered simply a cause of anemia and gallstone formation, has been shown to cause endothelial dysfunction and chronic vascular injury via the release of cell free plasma hemoglobin and arginase 1, which collectively reduce nitric oxide (NO) bioavailability and enhance reactive oxygen species (ROS) formation.2,3 Oxidation of hemoglobin can result in the release of free heme into plasma, which in excess has recently been shown to activate Toll-like receptor 4 (TLR4) and promote vasoocclusion and acute lung injury.4,5
Chen and colleagues show that, in addition to activating TLR4, heme promotes the release of NETs by increasing intracellular neutrophil ROS formation.1 NETs are decondensed chromatin, decorated with granular enzymes such as neutrophil elastase, that are released into the plasma by activated neutrophils.6 NETs are postulated to ensnare and kill pathogens with their high concentrations of granular enzymes. Chen and colleagues show that the production of NETs by neutrophils during tumor necrosis factor-α exposure promotes acute lung injury and death in sickle cell mice, akin to the toxicity of NETs observed in mouse models of transfusion-related acute lung injury.7
The increasing appreciation that intravascular hemolysis with release of hemoglobin and its oxidation products can drive sterile inflammation, via heme-TLR4 activation, suggests that erythrocyte hemolysis products can be considered damage-associated molecular pattern molecules (DAMPs). In this regard, they are similar to mitochondrial and cellular DNA, uric acid, adenosine, HMGB1, and other cytoplasmic and nuclear proteins that when released outside of the cell after tissue injury, cellular necrosis, and other stresses activate innate immunity and cause systemic inflammation in the absence of infection. DAMPs bind the same group of pattern recognition receptors, such as TLRs that mediate innate immunity to pathogens. Crystals of uric acid, a metabolic byproduct of DNA, can bind to the nucleotide-binding oligomerization domain–like receptors to potentially activate the NALP3 inflammasome and increase IL-1β production.8 Active erythropoiesis in the setting of hemolysis may generate excess uric acid from extruded red cell nuclei to activate this pathway. It is likely that systemic inflammation, oxidative stress, and infection in patients with sickle cell disease enhance the sensitivity of the innate immune system to erythroid DAMP molecules, such as extracellular heme.
The erythrocyte contains abundant antioxidant enzyme systems, such as super oxide dismutase, catalase, and the peroxiredoxins, and it forms a diffusional barrier that limits NO catabolism. Release of hemoglobin from erythrocytes during hemolysis sets in motion a cascade of molecular events that damage vascular endothelium and activate innate immune responses. Upstream NO reactions with oxyhemoglobin potently scavenge NO and inhibit its signaling.2 Oxidation of ferrous hemoglobin to ferric and feryl hemoglobin generate hydroxyl and lipid peroxyl radicals.3 These reactions promote vascular and renal injury, culminating in pulmonary hypertension and chronic kidney disease as patients age.9-11 Although haptoglobin and hemopexin limit the circulating levels of free hemoglobin and heme, the near saturation of these systems is evident among sickle cell disease patients in steady-state. These systems may become overwhelmed during the intensification of red cell hemolysis that often occurs during vasoocclusive painful crisis and acute chest syndrome.
The finding that hemolysis and heme play an important role in both TLR4 activation and NET formation in experimental models of vasoocclusion and acute chest syndrome opens the door to new therapeutic strategies to limit sterile inflammation in sickle cell disease patients. Chen et al highlight the therapeutic utility of DNase I treatment and neutrophil ROS scavenging with N-acetyl-cysteine. Upstream treatment with haptoglobin and hemopexin as well as downstream inhibition of TLR4 and the NALP3 inflammasome should be explored.
Conflict-of-interest disclosure: The authors declare no competing financial interests.