In this issue of Blood, Triot et al describe a novel genetic subtype of severe congenital neutropenia (SCN) characterized by inherited, biallelic loss-of-function mutations in the granulocyte–colony-stimulating factor (G-CSF) receptor gene, CSF3R.1 These findings expand the spectrum of pathogenic CSF3R mutations in humans and provide long overdue validation of mouse models generated in the 1990s.
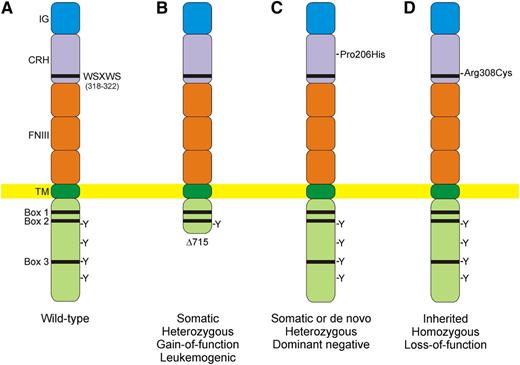
Acquired and inherited CSF3R mutations associated with SCN. (A) The extracellular portion of the wild-type G-CSF receptor contains conserved domains including immunoglobulin-like (IG), cytokine region of homology (CRH), and fibronectin type 3 (FNIII) domains. The WSXWS motif in the CRH domain acts as a molecular switch during receptor activation. Following ligand binding, 4 tyrosine (Y) residues in the cytoplasmic domain are rapidly phosphorylated, forming binding sites for intracellular signaling molecules. (B) Acquired gain-of-function mutations that truncate the cytoplasmic domain are common in patients with SCN and are associated with hyperproliferation and leukemic transformation. (C) Somatic or de novo heterozygous mutations affecting the extracellular domain act in a dominant negative fashion by inhibiting receptor trafficking to the cell surface and oligomerization in response to G-CSF. (D) Inherited, biallelic loss-of-function mutations cause a distinctive variant of SCN characterized by full myeloid maturation in the bone marrow and refractoriness to G-CSF therapy.
Acquired and inherited CSF3R mutations associated with SCN. (A) The extracellular portion of the wild-type G-CSF receptor contains conserved domains including immunoglobulin-like (IG), cytokine region of homology (CRH), and fibronectin type 3 (FNIII) domains. The WSXWS motif in the CRH domain acts as a molecular switch during receptor activation. Following ligand binding, 4 tyrosine (Y) residues in the cytoplasmic domain are rapidly phosphorylated, forming binding sites for intracellular signaling molecules. (B) Acquired gain-of-function mutations that truncate the cytoplasmic domain are common in patients with SCN and are associated with hyperproliferation and leukemic transformation. (C) Somatic or de novo heterozygous mutations affecting the extracellular domain act in a dominant negative fashion by inhibiting receptor trafficking to the cell surface and oligomerization in response to G-CSF. (D) Inherited, biallelic loss-of-function mutations cause a distinctive variant of SCN characterized by full myeloid maturation in the bone marrow and refractoriness to G-CSF therapy.
SCN is a heterogeneous group of disorders first identified in a consanguineous Swedish family by the Finnish physician Rolf Kostmann. Patients with SCN have severe neutropenia at birth with absolute neutrophil counts generally <500 cells/µL.2 In the majority of individuals with SCN, the bone marrow shows an arrest in myeloid maturation with a relative increase in promyelocytes or myelocytes.2 Serious bacterial infections, such as omphalitis, abscesses, and pneumonia, develop in 90% of patients with SCN by the age of 6 months.2
SCN exhibits multiple modes of inheritance, including autosomal dominant, autosomal recessive, X-linked, and sporadic forms.2 Heterozygous mutations in ELANE, encoding neutrophil elastase, account for 60% of the cases (exclusively autosomal dominant or sporadic forms).3 Biallelic mutations of the antiapoptotic gene HAX1 are associated with an autosomal recessive form of SCN (ie, Kostmann syndrome).3 Other genes mutated in SCN include G6PC3, GFI1, and the X-linked gene WAS.2
Historically, patients with SCN had a poor prognosis and generally died in the first decade of life due to overwhelming infection. The advent of recombinant G-CSF therapy altered the natural history of this disease by reducing the incidence and severity of bacterial infections.2 Despite G-CSF therapy, mortality due to infection remains a major problem in SCN, with a 12% cumulative incidence of death caused by sepsis by 15 years of age.4 Patients with SCN also have a markedly increased risk for myelodysplastic syndrome (MDS) and acute myelogenous leukemia (AML), with a cumulative incidence of 11% to 21% after 10 years.4 Patients with SCN who require high doses of recombinant G-CSF to maintain adequate neutrophil counts are at particular risk for leukemic transformation.
G-CSF augments the proliferation, differentiation, and survival of myeloid progenitors and the release of neutrophils into the circulation.5 G-CSF signals through its cognate receptor, G-CSFR, which forms homo-oligomeric complexes on ligand binding.5,6 Typical of other members of the type I cytokine receptor family, the extracellular domain of the G-CSFR contains a WSXWS motif that functions as a molecular switch during receptor activation (see panel A).7 The cytoplasmic domain of the receptor can be subdivided into a proximal domain, containing box 1 and box 2, that is essential for mitogenic signaling, and a distal domain, containing box 3, that is essential for the transduction of differentiation signals.6 The cytoplasmic domain of the receptor has no intrinsic kinase activity but activates Janus tyrosine kinases (JAKs) that transmit signaling via signal transducer and activator of transcription (STAT)3 and STAT5. Following receptor ligation, 4 tyrosine residues in the cytoplasmic region are rapidly phosphorylated, forming binding sites for intracellular signaling molecules.6
Mutations in the G-CSFR gene, CSF3R, were originally thought to be a cause of SCN.2,3 Subsequent studies, however, showed that the vast majority of CSF3R mutations observed in patients with SCN were somatic rather than inherited and were uniquely associated with the development of AML or MDS.2,3 These acquired heterozygous CSF3R mutations invariably introduce a premature stop codon that results in truncation of the distal cytoplasmic domain of the receptor and deranged signaling (see panel B). It is hypothesized that the loss of terminal differentiation signals from the distal cytoplasmic domain contributes to a hyperproliferative phenotype and leukemogenesis.8 In 1 published series, the incidence of cytoplasmic truncation mutations was 78% in individuals with SCN and MDS or AML vs 34% in individuals without signs of malignant transformation.9
In rare instances, somatic or de novo heterozygous mutations affecting the extracellular domain of the G-CSFR have been reported to cause sporadic SCN (see panel C).10 Such mutations are thought to act in a dominant negative fashion by inhibiting receptor trafficking to the cell surface and oligomerization in response to G-CSF.10 These variants of SCN are unresponsive to high doses of recombinant G-CSF.
Now Triot et al identify a new subtype of SCN, characterized by recessively inherited, loss-of-function mutations in CSF3R, in 2 unrelated families.1 The affected children in the first family harbored a homozygous missense mutation p.Arg308Cys, near the WSXWS motif (see panel D), that resulted in abnormal G-CSFR glycosylation, impaired trafficking of the receptor to the cell surface, and reduced downstream signaling. Paralogous disease-causing mutations have been reported in other type I cytokine receptors.1 The affected child in the second family carried compound heterozygous frameshift mutations that truncated the receptor in the extracellular domain (p.Gly316fsTer322 and p.Gly415fsTer432). None of the affected children in either kindred responded to treatment with recombinant G-CSF.
Despite reduced numbers of circulating neutrophils, all the patients had morphological evidence of full myeloid cell maturation in the bone marrow. This contrasts with SCN caused by ELANE or HAX1 mutations, wherein an arrest in myeloid maturation is pathognomonic. Like the patients reported by Triot et al, mice harboring homozygous loss-of-function mutations in either G-CSF or its receptor have reduced numbers of neutrophils in the peripheral blood and full myeloid maturation in the bone marrow.5,6 Altogether, these studies in humans and mice support the existence of G-CSFR-independent signaling pathways that control myelopoiesis. Remarkably, it has taken 2 decades of analyzing patient samples to confirm predictions made on the basis of these genetically engineered mouse models, underscoring the premise that validation is a dish oft served cold.
In 20% to 30% of SCN cases, a genetic cause has not yet been identified. In the coming years, the molecular basis for other rare subtypes of SCN may be elucidated and shed light on the G-CSFR-independent signaling pathways that regulate granulopoiesis. The generation of induced pluripotent stem cells (iPSCs) from patients harboring biallelic loss-of-function mutations in CSF3R, such as those described by Triot et al, would facilitate the study of G-CSFR-independent myelopoiesis. Eventually, advances in genome sequencing technology and iPSC differentiation models may obviate the need for mouse models and their validation.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal