Key Points
Sickle cell patients show mitochondrial dysfunction (complex V inhibition, oxidant formation), which is associated with platelet activation.
Complex V inhibition is induced by hemolysis and causes platelet activation, which is attenuated by mitochondrial therapeutics.
Abstract
Bioenergetic dysfunction, although central to the pathogenesis of numerous diseases, remains uncharacterized in many patient populations because of the invasiveness of obtaining tissue for mitochondrial studies. Although platelets are an accessible source of mitochondria, the role of bioenergetics in regulating platelet function remains unclear. Herein, we validate extracellular flux analysis in human platelets and use this technique to screen for mitochondrial dysfunction in sickle cell disease (SCD) patients, a population with aberrant platelet activation of an unknown mechanism and in which mitochondrial function has never been assessed. We identify a bioenergetic alteration in SCD patients characterized by deficient complex V activity, leading to decreased mitochondrial respiration, membrane hyperpolarization, and augmented oxidant production compared with healthy subjects. This dysfunction correlates with platelet activation and hemolysis in vivo and can be recapitulated in vitro by exposing healthy platelets to hemoglobin or a complex V inhibitor. Further, reproduction of this dysfunction in vitro activates healthy platelets, an effect prevented by attenuation of mitochondrial hyperpolarization or by scavenging mitochondrial oxidants. These data identify bioenergetic dysfunction in SCD patients for the first time and establish mitochondrial hyperpolarization and oxidant generation as potential pathogenic mechanism in SCD as well as a modulator of healthy platelet function.
Introduction
The mitochondrion is an integral regulator of cellular function in most cell types. Beyond maintenance of energy homeostasis, the electron transport chain (ETC) regulates cellular fate through the initiation of apoptosis and dynamically produces reactive oxygen species (ROS) to mediate redox signaling. Although it is now well established that altered bioenergetics contribute to the pathogenesis of a wide range of diseases in which the primary cause is nonmitochondrial, the exact function of the mitochondrion in many cell types, particularly circulating cells, remains elusive. Further, bioenergetics remains uncharacterized in many patient populations because of the requirement for viable intact human tissue to accurately measure mitochondrial function.
Platelets are an easily accessible source of human mitochondria, and prior studies have measured ETC function in these thrombotic mediators as a surrogate for bioenergetic function in other organs.1,2 Identification of specific mitochondrial alterations in platelets from patients with a variety of pathologies including Parkinson disease,1,3,4 sepsis,2,5 and type 2 diabetes melllitus6,7 have established that platelets can be used as biomarkers for systemic mitochondrial dysfunction. However, the exact role of bioenergetics in regulating platelet thrombotic function is less clear. Studies of healthy platelets show that mitochondria supply a fraction of the ATP required for α-granule secretion during platelet aggregation.8,9 In addition, the loss of mitochondrial membrane potential (ΔΨ) and increased membrane permeability initiate platelet phosphatidyl serine exposure and regulate coagulation.10 Emerging in vitro data now suggest a role for augmented ΔΨ in regulating platelet sensitivity to thrombotic stimuli.11,12 However, the contribution of mitochondrial hyperpolarization to platelet activation in a patient population with known platelet dysfunction has not been assessed.
Sickle cell disease (SCD) is a homozygous recessive disorder caused by a single-point mutation in the β-globin chain of hemoglobin A, resulting in mutant hemoglobin (HbS). Although the primary dysfunction in SCD patients is the hypoxic polymerization of HbS, leading to diminished erythrocyte deformability and impaired microvascular blood flow, it is well documented that these patients demonstrate characteristics of chronic hemostatic activation, including elevated levels of platelet activation.13-16 Although the molecular mechanism underlying this platelet dysfunction is unknown, platelet activation is associated with augmented erythrocytic hemolysis in these patients.15 Clinically, platelet activation contributes to both acute and chronic vascular complications, including vaso-occlusive crisis and pulmonary arterial hypertension (PAH), through the secretion of vasoactive and mitogenic factors.13,14,17-19 Notably, although aberrations in mitochondrial redox signaling and bioenergetics have been implicated in the pathogenesis of both systemic20 and pulmonary21 vasculopathies, mitochondrial function has never been assessed in SCD patients.
The advent of extracellular flux (XF) analysis has enabled the high throughput assessment of bioenergetics in small numbers of intact live cells, and this technology has recently been applied to human platelets.6,22,23 Herein, we further validate XF analysis and couple this technology with biochemical measures of mitochondrial ROS generation and platelet thrombotic function to screen SCD patients for bioenergetic dysfunction and simultaneously determine the contribution of the mitochondrial ETC to platelet activation in this population. We identify a specific bioenergetic alteration in SCD patients and provide in vivo and in vitro evidence that this specific alteration in the mitochondrial ETC may mechanistically underlie augmented platelet activation in this population. The implications of this data for normal platelet physiology, as well as the mitochondrion as a potential SCD therapeutic target, will be discussed.
Methods
Materials
Chemicals were purchased from Sigma-Aldrich (St. Louis, MO) and antibodies from Becton Dickinson (San Jose, CA) unless otherwise noted.
Study population
This study was approved by the institutional review board of the University of Pittsburgh Medical Center (UPMC), and written informed consent was obtained from all participants in accordance with the Declaration of Helsinki. The study group consisted of 24 adult patients with homozygous SCD (HbSS) in steady state selected from the UPMC hematology clinic and 19 African-American control participants with no known hemoglobinopathy. None of the participants was taking anticoagulant/antiplatelet medication or received transfusions in the 3 months before blood draw. Laboratory characteristics are summarized in Table 1.
Platelet isolation
Venous blood samples were collected in citrate by standard venous puncture, and the first 2 mL of blood was discarded to avoid artificial activation. Platelets were isolated by differential centrifugation as previously described.15 Briefly, whole blood was centrifuged (150g for 10 minutes) in the presence of PGI2 (1 µg/mL) to obtain platelet-rich plasma (PRP). Platelets were subsequently pelleted by centrifugation (1500g for 10 minutes). The platelets were washed in erythrocyte lysis buffer containing PGI2, and final samples were resuspended in modified Tyrode buffer (20 mmol/L HEPES, 128 mmol/L NaCl, 12 mmol/L bicarbonate, 0.4 mmol/L NaH2PO2, 5 mmol/L glucose, 1 mmol/L MgCl2, 2.8 mmol/L KCl, pH 7.4). Platelet purity was confirmed by flow cytometric measurement of CD41a expression.
Platelet activation
Whole blood or platelets were incubated with phycoerythrin (PE)-labeled mouse anti-human CD41a antibody and APC-labeled mouse anti-human CD62P antibody (30 min; 25°C) to measure surface p-selectin expression by flow cytometry (LSRFortessa with FASCDiva software; Becton Dickinson). FITC-labeled PAC-1 was used in some studies to recognize activated glycoprotein IIb/IIIa, and data are expressed as a percentage of total integrin binding. Platelets were identified by their characteristic light scatter and CD41a antibody binding. Activated platelets are reported as the percentage of 10 000 CD41+ platelets exhibiting APC-CD62P fluorescence.
Platelet aggregation
Platelet number was normalized (108) in PRP and then the sample was treated with hemoglobin or ADP before aggregation was followed for 15 minutes using a single-channel aggregometer (Chrono-Log Corp). All data are expressed in percent aggregation at 15 minutes and are normalized to a standard deflection, corresponding to light transmission through platelet-poor plasma.
Platelet factor 4 release
Healthy human platelets in Tyrode buffer were untreated or treated with ferrous human hemoglobin for 30 minutes and then the buffer subjected to enzyme-linked immunosorbent assay (Sigma-Aldrich) to measure the release of human platelet factor 4.
Oxygen consumption rate
Platelet number was determined spectrophotometrically at 800 nm as described in Walkowiak et al.24 Platelets (50 million/well) were loaded in unbuffered Dulbecco’s Modified Eagle Medium (DMEM) into each well of an XF24 microplate to measure oxygen consumption rate (OCR) by XF analysis (XF24, Seahorse Biosciences, Billerica, MA). The plate was subsequently centrifuged (1500g for 4 minutes) to form a monolayer in the well. XF analysis commenced after equilibration of the plate (10 minutes; 37°C). For bioenergetic profile measurement, platelets were consecutively treated with oligomycin A (2.5 µmol/L), carbonyl cyanide p-(trifluoro-methoxy) phenyl-hydrazone (FCCP; 0.7 µmol/L), and Rotenone (10 µmol/L). Optimal concentration of each modulator was determined in concentration response experiments. For measurement of OCR by Clark electrode, isolated platelets were suspended in unbuffered DMEM in a Clark-type electrode containing chamber (Instech, Plymouth Meeting, PA) and OCR measured in the absence and presence of FCCP (0.7 µmol/L).
Measurement of ΔΨ
Platelets were incubated with 500 nmol/L JC-1 (Molecular Probes, Eugene, CA) for 5 minutes and then subjected to 2-color flow cytometry to quantify the proportion of cells that contain JC-1 green monomer (indicative of low ΔΨ) or red dimer (indicative of hyperpolarization). The ratio of red-to-green fluorescence was calculated and expressed on a percentage scale in which 100% was oligomycin (0.7 µmol/L)-treated platelets for maximal ΔΨ, and 0% was FCCP (0.7 µmol/L)-treated platelets.
Mitochondrial ROS generation
Platelets were incubated with MitoSOX (Invitrogen, Carlsbad, CA; 5 µM, 10 minutes) and washed, and fluorescent intensity (510/580 nm) was measured kinetically. In select experiments, H2O2 generation was quantified by measuring the rotenone-sensitive oxidation of Amplex Red as previously described.25
ETC complex expression and activity assays
Complex expression was measured by western blot. Antibodies to mitochondrial ETC and integrin αIIb were purchased from Mitosciences (Eugene, OR) and Santa Cruz Biotech (Dallas, TX), respectively. Densitometry was performed on a LICOR system and complex expression normalized to integrin αIIb. The enzymatic activity of complexes I-V and citrate synthase were measured by spectrophotometric kinetic assays after several cycles of platelet freeze/thaw as previously described.25
Isolation of human hemoglobin
Measurement of plasma hemoglobin
The concentration of free hemoglobin in SCD plasma was measured by a chemiluminescence nitric oxide (NO) analyzer (GE Analytical) by assessing the consumption of NO by plasma samples and compared with a standard curve derived from the consumption of NO by known concentrations of hemoglobin as previously described.27
Statistics
Individual group samples were compared by Student t test, whereas multiple comparisons were made by appropriate analysis of variance. Correlations were performed by 2-tailed nonparametric Spearman correlations (because of non-Gaussian distribution of data) and linear regression analysis with 95% confidence interval; P <.05 was considered significant. Data are presented as the means ± standard error of the mean (SEM) unless otherwise specified.
Results
Validation of human platelet bioenergetic measurements by XF analysis
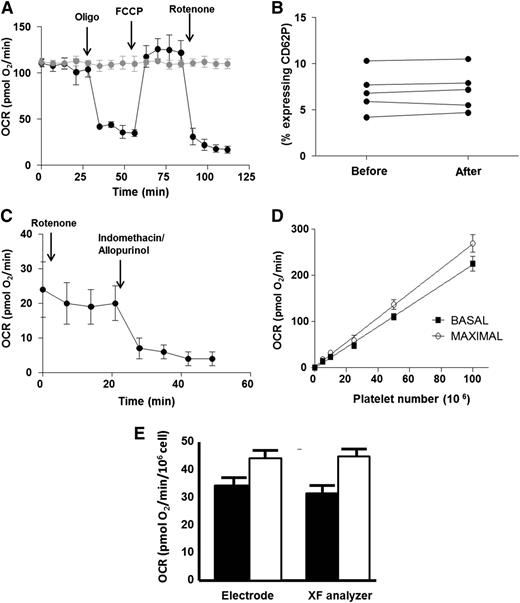
We first sought to validate the use of XF analysis as a screening tool for human platelet bioenergetics. Platelets were isolated from a fresh blood draw from healthy African-American subjects and seeded for XF analysis at a density of 50 × 106 platelets per well. Measurement of OCR in the absence of treatment showed that basal OCR was stable for 2 hours (Figure 1A). Further, measurement of platelet activation before and after XF analysis demonstrated that seeding and XF measurement did not stimulate platelet activation (Figure 1B). To generate a bioenergetic profile, basal OCR was first measured. The complex V inhibitor oligomycin (2.5 µmol/L) was then administered to quantify the OCR not linked to ATP synthesis. The resulting rate is indicative of proton leak across the inner mitochondrial membrane. Next, the uncoupler FCCP (0.7 µmol/L) was added to stimulate the maximal respiratory rate. Finally, the complex I inhibitor, rotenone (10 µmol/L), was administered to determine the nonmitochondrial OCR by the platelets. Consistent with prior studies,6,22 platelets responded to all pharmacologic agents, demonstrating fully functional mitochondria and generating a measurable bioenergetic profile. Notably, platelets possessed a significant rate of nonmitochondrial OCR (Figure 1A,C). This nonmitochondrial OCR was inhibited by treatment of the platelets with allopurinol (100 µmol/L) and indomethacin (100 µmol/L), inhibitors of the oxygen-consuming enzymes xanthine oxidoreductase and cyclooxygenase, respectively (Figure 1C).
Validation of XF analysis for human platelets. (A) Typical bioenergetic profile of platelets from a healthy African-American subject. Arrows denote the addition of oligomycin (2.5 µmol/L), FCCP (0.7 µmol/L), and rotenone (10 µmol/L) to black trace. Gray trace is the same sample in the absence of modulators. All OCR rates are normalized to 106 platelets. (B) Percent of activated platelets measured in 5 different samples before seeding in the XF analyzer and after measurement. (C) OCR of healthy platelets in the presence of rotenone. Arrows denote the addition of indomethacin (100 µmol/L) and allopurinol (100 µmol/L). (D) Basal (black squares) and maximal (open circles) OCR in increasing numbers of healthy human platelets. (E) Basal (black bars) and maximal (white bars) OCR in healthy platelets measured by XF analysis versus Clark electrode.
Validation of XF analysis for human platelets. (A) Typical bioenergetic profile of platelets from a healthy African-American subject. Arrows denote the addition of oligomycin (2.5 µmol/L), FCCP (0.7 µmol/L), and rotenone (10 µmol/L) to black trace. Gray trace is the same sample in the absence of modulators. All OCR rates are normalized to 106 platelets. (B) Percent of activated platelets measured in 5 different samples before seeding in the XF analyzer and after measurement. (C) OCR of healthy platelets in the presence of rotenone. Arrows denote the addition of indomethacin (100 µmol/L) and allopurinol (100 µmol/L). (D) Basal (black squares) and maximal (open circles) OCR in increasing numbers of healthy human platelets. (E) Basal (black bars) and maximal (white bars) OCR in healthy platelets measured by XF analysis versus Clark electrode.
Basal and maximal respiratory rates were dependent on platelet seeding density and showed a linear response between 5 to 100 × 106 platelets per well (Figure 1D). To compare the accuracy of XF analysis with a conventional method of respirometry, basal and maximal OCR were measured by a Clark-type oxygen electrode and XF analysis in platelets isolated from the same person. Although no significant difference was found in the basal and maximal OCR obtained by the differing methods when normalized to platelet number, measurement by conventional electrode required significantly greater numbers of platelets (8 × 108) than XF analysis (25 × 106) to obtain reliable rates (Figure 1E).
SCD patient platelets have altered bioenergetics
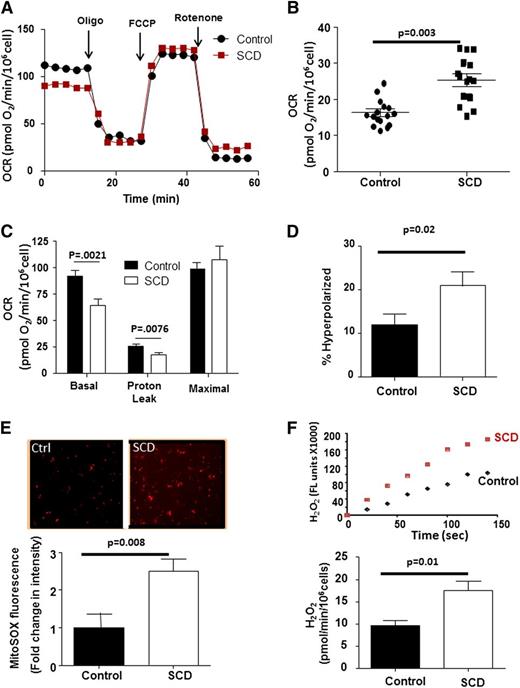
We next used platelet XF analysis to screen SCD patients (n = 24) for altered bioenergetics compared with healthy African-American control subjects (n = 19) (Figure 2A and Table 1). Although platelets from both groups demonstrated robust responses to each mitochondrial modulator, SCD patient platelets exhibited a significantly greater nonmitochondrial OCR (25.3 ± 1.9 pmol/min/106 cells) than controls (16.4 ± 1.1 pmol/min/106 cells; Figure 2B). This rotenone-insensitive OCR was completely inhibited by allopurinol (100 µM) and indomethacin (100 µM; data not shown), consistent with prior reports of increased activity of oxygen-consuming enzymes in SCD.28 When normalized for differences in nonmitochondrial OCR, platelets from SCD patients showed a significantly lower rate of basal respiration in (64 ± 6.5 pmol/min), as well as decreased OCR caused by proton leak (17.4 ± 2.2 pmol/min) than healthy subjects (92.3 ± 5.1 and 25.7 ± 1.9 pmol/min), with no significant difference in maximal OCR (99.9 ± 6.5 vs 107 ± 13 pmol/min) (Figure 2C).
SCD platelets show bioenergetic dysfunction. (A) Representative platelet bioenergetic profile from an SCD patient (red) and healthy control (black). (B) Rotenone-insensitive OCR of control and SCD patient platelets. Bars represent mean ± SEM. (C) Quantification of each OCR component of bioenergetic profile of platelets for an SCD patient and control after correction for nonmitochondrial OCR (n = 24 SCD; 19 controls). (D) Quantification of the percent of hyperpolarized mitochondria in platelets from SCD and control subjects (n = 16 each group). (E) Representative MitoSOX fluorescence images from control and SCD platelets and relative fluorescence intensity of MitoSOX labeling in control and SCD platelets (n = 18 each group). (F) Representative Amplex Red traces and quantification of H2O2 generation (normalized to 106 cells) from control and SCD platelets (n = 14 each).
SCD platelets show bioenergetic dysfunction. (A) Representative platelet bioenergetic profile from an SCD patient (red) and healthy control (black). (B) Rotenone-insensitive OCR of control and SCD patient platelets. Bars represent mean ± SEM. (C) Quantification of each OCR component of bioenergetic profile of platelets for an SCD patient and control after correction for nonmitochondrial OCR (n = 24 SCD; 19 controls). (D) Quantification of the percent of hyperpolarized mitochondria in platelets from SCD and control subjects (n = 16 each group). (E) Representative MitoSOX fluorescence images from control and SCD platelets and relative fluorescence intensity of MitoSOX labeling in control and SCD platelets (n = 18 each group). (F) Representative Amplex Red traces and quantification of H2O2 generation (normalized to 106 cells) from control and SCD platelets (n = 14 each).
Because decreased proton leak can result in increased ΔΨ, we next assessed ΔΨ and found that platelets from SCD patients were significantly more hyperpolarized than those from controls (Figure 2D). Mitochondrial hyperpolarization can result in increased ETC reduction, propagating electron leak and subsequent oxidant generation. Using mitoSOX, we next demonstrated that mitochondria in platelets from SCD patients generated ∼2.4-fold greater concentrations of mitoSOX oxidation products than healthy platelets, consistent with increased mitochondrial oxidant production (Figure 2E). Measurement of Amplex Red oxidation showed that SCD platelets generated significantly more hydrogen peroxide (H2O2) than controls (Figure 2F).
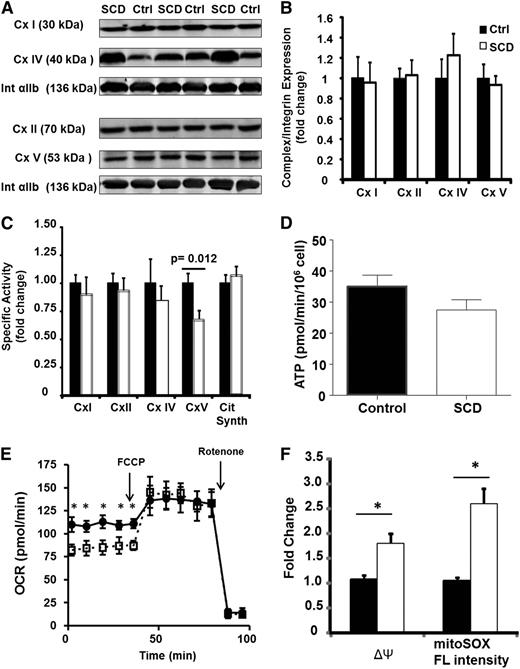
To determine whether these bioenergetic and redox changes were caused by a specific enzymatic alteration, we next examined individual enzymatic components of the ETC in platelets from SCD patients and controls. Although the protein expression of complexes I, II, IV, and V were similar in both groups (Figure 3A-B), the specific enzymatic activity of complex V was significantly decreased in SCD patients vs controls (16.7 ± 2.3 vs 25.2 ± 2.5 nmol/min/mg) (Figure 3C). Notably, although complex V activity was decreased, platelet ATP generation rate was not significantly different (Figure 3D). This was potentially the result of a statistically significant (P < .05) upregulation of glycolysis (measured as the extracellular acidification rate) in the platelets isolated from SCD patients (5.4 ± 0.9 mpH/min/106 cells) compared with that of healthy controls (3.2 ± 0.4 mpH/min/106 cells).
SCD platelets have inhibited complex V activity. (A) Representative western blots for complexes I, II, IV, and V and integrin αIIb in 3 control and 3 SCD subjects. (B) Densitometric quantification of several such blots in 12 control and SCD platelets. (C) Enzymatic activity of each ETC complex in platelets from SCD patients (black bars) and healthy controls (white bars; n = 15). (D) ATP generation rate in SCD (black bars) and control (white bars) platelets (n = 10 each). (E) Bioenergetic profile (basal and FCCP-induced OCR) for untreated (filled circles) or oligomycin treated (open squares) platelets. (F) ΔΨ and MitoSOX fluorescence intensity of untreated (black bars) or oligomycin-treated (white) healthy platelets (n = 5, *P < .01).
SCD platelets have inhibited complex V activity. (A) Representative western blots for complexes I, II, IV, and V and integrin αIIb in 3 control and 3 SCD subjects. (B) Densitometric quantification of several such blots in 12 control and SCD platelets. (C) Enzymatic activity of each ETC complex in platelets from SCD patients (black bars) and healthy controls (white bars; n = 15). (D) ATP generation rate in SCD (black bars) and control (white bars) platelets (n = 10 each). (E) Bioenergetic profile (basal and FCCP-induced OCR) for untreated (filled circles) or oligomycin treated (open squares) platelets. (F) ΔΨ and MitoSOX fluorescence intensity of untreated (black bars) or oligomycin-treated (white) healthy platelets (n = 5, *P < .01).
Complex V is a point of proton reentry from the mitochondrial intermembrane space to the matrix, and inhibition of this activity could decrease basal OCR and increase ΔΨ and ROS generation. To test whether this occurs in platelets, healthy human platelets were treated in vitro with a low dose of oligomycin (0.4 µmol/L), which inhibited complex V activity by 30 ± 7%. Measurement of OCR showed that this treatment decreased basal OCR while having no effect on maximal OCR (Figure 3E). Further, ΔΨ and oxidant production were augmented by 1.6 ± 0.1-fold and 2.1 ± 0.2-fold, respectively (Figure 3F), generating a similar profile to that observed in SCD platelets. Collectively, these data demonstrate that SCD patients harbor a bioenergetic aberrancy characterized by decreased respiration, increased ΔΨ, and augmented mitochondrial oxidant generation, and this is potentially caused by deficient complex V activity.
Mitochondrial dysfunction is associated with platelet activation and hemolysis
SCD patients exhibit augmented platelet activation compared with healthy subjects.13-15 This phenomenon was corroborated in our cohort in which SCD patient platelets showed a significantly greater percentage of activated platelets (21 ± 6.9%; n = 24) than controls (11.0 ± 2.3%, n = 19, P = .029; Table 1). Thus, we next sought to determine whether the bioenergetic dysfunction identified in SCD platelets was associated with aberrant platelet activation. We found a significant positive correlation between 2 markers of platelet activation (surface p-selectin and activated glycoprotein IIb/IIIa) and the degree of mitochondrial hyperpolarization or H2O2 production in SCD patients (Table 2 and supplemental Figure 1). These data suggested that platelet mitochondrial dysfunction is associated with increased platelet activation in SCD.
SCD patients have high rates of intravascular hemolysis, and this hemolysis has been associated with platelet activation in these individuals.15,29 Consistent with published studies, a significant positive correlation was found between the concentration of plasma-free hemoglobin and the percentage of activated platelets (surface p-selectin expression) in our SCD cohort (r = 0.49; P = .04). Notably, strong correlations were also observed between all markers of mitochondrial dysfunction (ΔΨ, H2O2 production, and complex V activity) and plasma cell–free hemoglobin (a marker of hemolysis) in these subjects (Table 3 and supplemental Figure 2). Collectively, these data suggested an association among hemolysis, mitochondrial dysfunction, and platelet activation in SCD patients.
Bioenergetic dysfunction: a mechanistic link between hemolysis and platelet activation
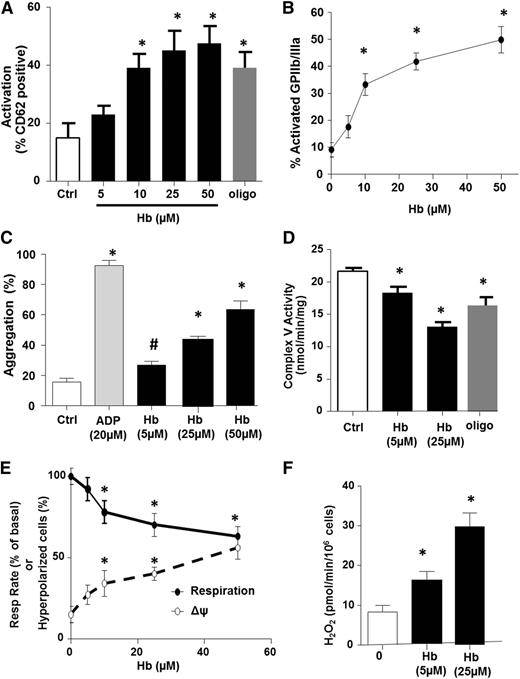
We next performed a series of in vitro experiments to determine whether hemolysis causes the platelet mitochondrial dysfunction and platelet activation observed in SCD patients. Healthy human PRP was exposed to increasing concentrations of oxygenated hemoglobin (0-50 µmol/L), and platelet mitochondrial function was measured concomitantly with platelet thrombotic function. Consistent with prior studies,15 platelet activation was significantly increased after hemoglobin exposure (Figure 4A-B). Further, hemoglobin induced platelet aggregation in a concentration-dependent manner, although to a much lesser extent than 20 µM of the classic agonist ADP (Figure 4C). Hemoglobin-mediated stimulation of α-granule secretion was also confirmed by the measurement of a 2.1 ± 0.3-fold increase in platelet factor 4 release by the hemoglobin-treated platelet compared with untreated controls. Measurement of complex V activity and bioenergetics in these platelets showed a hemoglobin-dependent decrease in complex V activity (Figure 4D) and basal OCR (Figure 4E), as well as an increase in ΔΨ (Figure 4E) and H2O2 production (Figure 4F), recapitulating the bioenergetic alteration observed in SCD patients. Importantly, partial inhibition of complex V with oligomycin (0.4 µmol/L; Figure 4D) also resulted in platelet activation (Figure 4A). These data demonstrate that partial inhibition of complex V pharmacologically or by hemoglobin exposure is sufficient to increase ΔΨ, augment ROS generation, and activate platelets.
Hemolysis induces platelet aggregation and mitochondrial dysfunction. (A-D) Platelet activation measured by surface p-selectin expression (A) or percent of activated glycoprotein IIb/IIIa (B), platelet aggregation 15 minutes after treatment (C), and complex V activity (D) in untreated (control) healthy platelets or those exposed to free hemoglobin or oligomycin (0.4 µmol/L) or ADP (20 µM, to stimulate aggregation). (E) Basal respiratory rate (solid line) and mitochondrial hyperpolarization (dashed line) in healthy platelets exposed to increasing concentrations of free hemoglobin. (F) H2O2 generation in hemoglobin-treated platelets (n = 5, *P < .01 vs untreated).
Hemolysis induces platelet aggregation and mitochondrial dysfunction. (A-D) Platelet activation measured by surface p-selectin expression (A) or percent of activated glycoprotein IIb/IIIa (B), platelet aggregation 15 minutes after treatment (C), and complex V activity (D) in untreated (control) healthy platelets or those exposed to free hemoglobin or oligomycin (0.4 µmol/L) or ADP (20 µM, to stimulate aggregation). (E) Basal respiratory rate (solid line) and mitochondrial hyperpolarization (dashed line) in healthy platelets exposed to increasing concentrations of free hemoglobin. (F) H2O2 generation in hemoglobin-treated platelets (n = 5, *P < .01 vs untreated).
Attenuation of hyperpolarization or ROS scavenging prevents platelet activation
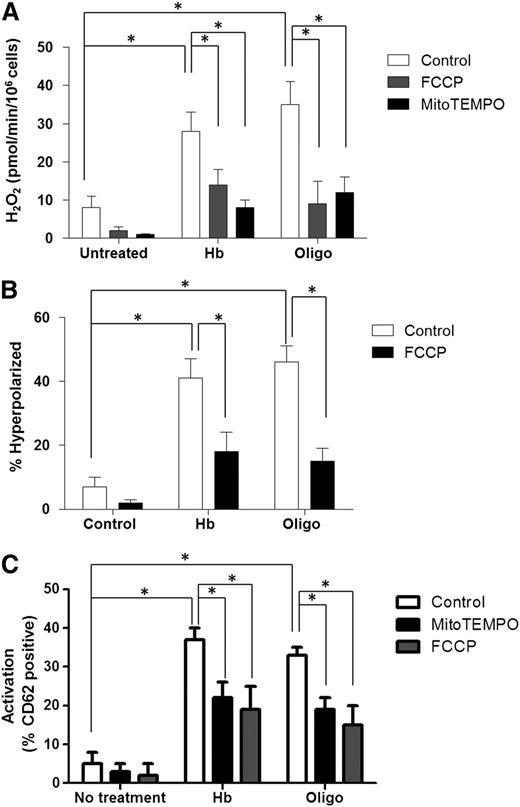
To directly determine whether mitochondrial hyperpolarization and H2O2 generation induced by complex V inhibition causes platelet activation, healthy human PRP was incubated with hemoglobin (25 µmol/L) or oligomycin (0.4 µmol/L) in the absence and presence of the mitochondrially-targeted ROS scavenger mitoTEMPO (100 µmol/L), or a low concentration of the mitochondrial uncoupler FCCP (0.3 µmol/L), to partially decrease ΔΨ. As expected, MitoTEMPO treatment had no effect on ΔΨ (not shown) but significantly decreased H2O2 concentration (Figure 5A). Consistent with the fact that hyperpolarization induces ROS generation, partial attenuation of ΔΨ by FCCP (Figure 5B) also decreased hemoglobin or oligomycin-dependent H2O2 generation (Figure 5A). Importantly, both mitoTEMPO and FCCP significantly decreased oligomycin or hemoglobin-induced platelet activation (Figure 5C). Collectively, these data demonstrate that complex V inhibition leading to mitochondrial hyperpolarization induces subsequent ROS production, which ultimately causes platelet activation.
Mitochondrial uncoupling and ROS scavenging attenuates platelet activation. H2O2 production (A), ΔΨ (B), and activation levels (C) in untreated (control) healthy platelets or those exposed to hemoglobin (25 µmol/L) or oligomycin (0.4 µmol/L) in the absence (white bars) or presence of FCCP (0.35 µmol/L; gray bars), or mitoTEMPO (100 µmol/L; black bars). Data are presented as mean ± SEM (n > 4; P < .01).
Mitochondrial uncoupling and ROS scavenging attenuates platelet activation. H2O2 production (A), ΔΨ (B), and activation levels (C) in untreated (control) healthy platelets or those exposed to hemoglobin (25 µmol/L) or oligomycin (0.4 µmol/L) in the absence (white bars) or presence of FCCP (0.35 µmol/L; gray bars), or mitoTEMPO (100 µmol/L; black bars). Data are presented as mean ± SEM (n > 4; P < .01).
Discussion
This study represents the first direct examination of mitochondrial function in SCD patients and provides evidence that bioenergetic dysfunction mechanistically contributes to SCD-induced platelet activation. The data herein identify a specific bioenergetic alteration in this cohort induced by free hemoglobin, characterized by inhibited complex V activity, which leads to increased ΔΨ and augmented oxidant generation. This bioenergetic dysfunction is associated with enhanced platelet activation in vivo. Further, partial inhibition of complex V in healthy platelets in vitro recapitulates the bioenergetic dysfunction observed in SCD patients and results in platelet activation, establishing a causal relationship between this bioenergetic alteration and platelet activation.
The present study suggests that mitochondrial dysfunction, through the stimulation of platelet activation, potentially contributes to SCD-induced vascular pathogenesis. In addition to overt thrombus formation, activated platelets contribute to both acute and chronic vasculopathy in SCD patients through the secretion of soluble vasoactive and mitogenic factors, promoting vascular damage, red cell adhesion to the endothelium, and intimal hyperplasia.13,14,17-19 Consistent with this, augmented platelet activation observed basally in steady-state SCD patients is further elevated during acute vaso-occlusive crisis and correlates with the severity of PAH, a chronic vascular proliferative complication and leading cause of mortality in adult SCD patients.14-16,30-32 Although patients in vaso-occlusive crisis or with diagnosed PAH were excluded from this study, it will be important to determine whether complex V inhibition and the bioenergetic dysfunction observed is further exacerbated in these populations. It is also unclear whether the mitochondrial bioenergetic dysfunction is reversible; however, the in vitro data presented show that scavenging of mitochondrial ROS or mild attenuation of ΔΨ prevents hemoglobin-induced platelet activation. Recent clinical trials have demonstrated beneficial effects of antiplatelet therapies on pain events in SCD patients.33 Although mitochondria have previously not been considered a therapeutic target in SCD, it is intriguing to speculate whether existing strategies to decrease mitochondrial ROS, such as administration of the mitochondrial antioxidant MitoQ,34 may have favorable effects on decreasing pain and decelerating PAH and thrombotic development in SCD.
Our data demonstrate that hemoglobin induces a shift in mitochondrial function, resulting in the generation of oxidants. Notably, although oxidant production is increased by in vitro hemoglobin treatment and in SCD patients, ATP generation is not compromised. Comparison of H2O2 generation in control and SCD platelets shows that ∼11% of basal oxygen consumption in control platelets contributes to H2O2 production, whereas in SCD platelets, H2O2 accounts for ∼20%. Interestingly, although significantly more oxygen consumption is diverted to ROS generation in SCD platelets, ATP generation does not decrease significantly. Calculation of the phosphate:oxygen (P:O) ratio in the 2 platelet populations shows a trend for increased efficiency in platelets from SCD patients compared with controls (2.96 ± 0.36) compared with controls (2.25 ± 0.29). Although the mechanism underlying this maintenance of ATP generation is not completely clear, upregulation of glycolysis could be a contributor. Further, this type of shift may represent a signaling mechanism by which the mitochondrion generates ROS to modulate signaling outside the mitochondrion. Future studies will determine whether mitochondrial ROS contribute to platelet function beyond activation and aggregation.
It is also conceivable that the platelets serve as a surrogate for mitochondria in other cell types,1,35 and this type of metabolic shift underlies mitochondrial redox signaling in the vasculature. For example, in the context of SCD, mitochondrial H2O2 generation in vascular endothelial and smooth muscle cells has been implicated in pulmonary vascular remodeling.21,36 Further, mitochondrial H2O2 mediates the hypoxic stabilization of hypoxia-inducible factor 1α (HIF-1α),37 which could contribute to the aberrant normoxic stabilization of HIF-1α, which has been reported in SCD and is implicated in the pathogenesis of vasculopathy.38-40
Hemolysis and platelet activation are known to be closely associated in SCD patients.14,15,29 The current study confirms this relationship and extends it to include mitochondrial dysfunction as a mechanistic link. Although our data demonstrate that exposure of healthy platelets to hemoglobin in vitro mimics the mitochondrial dysfunction observed in SCD platelets, it is unclear how hemolysis inhibits complex V function. No change in complex V protein expression was observed in SCD patients, suggesting that heme-induced posttranslational modification is likely responsible for this change in activity. Although a number of posttranslational modifications have been demonstrated to regulate complex V activity in other cell types, reports of enzymatic inhibition by nitration, S-nitrosation, and cysteine oxidation are particularly relevant in the context of platelets and hemolysis.41,42 The ferrous (Fe2+) heme contained in cell-free hemoglobin is both a potent scavenger of nitric oxide (NO)43 and a catalyst for the generation of oxidants.44,45 Prior studies have focused on the role of hemoglobin-mediated NO scavenging in hemolysis-induced platelet activation.15 However, it is conceivable that in the presence of superoxide generated by free hemoglobin, NO could be oxidized to yield species such as nitrogen dioxide (NO2●) and peroxynitrite (ONOO–) that mediate nitration or S-nitrosation of complex V, which results in its inhibition.41 Current studies are aimed at investigating the mechanism of hemoglobin-induced mitochondrial dysfunction, and it will be important to determine whether this mechanism is present in hemolytic disorders beyond SCD.
Although the precise signaling pathway between mitochondrial ROS and platelet activation is unknown, several studies support the role of oxidants in activating platelets.10-12,46,47 Specifically, collagen-induced platelet activation has been shown to involve the production of H2O2, which modulates activation of the phospholipase C–signaling cascade.47 Further, mitochondrial ROS generation sensitizes platelets to activation mediated by other physiologic stimuli.11,12 However, the role of mitochondrial ΔΨ in regulating platelet activation is less clear. Platelet activation has been linked to both mitochondrial hyperpolarization and depolarization.10-12,48 This discrepancy is likely a result of differences in time course or strength of stimulation across studies, as demonstrated by a study in which stimulation of platelets with opsonized zymosan–induced mitochondrial hyperpolarization stimulated platelet activation followed temporally by depolarization, leading to phosphatidyl serine externalization.11 On the basis of this idea, it is conceivable that small dynamic changes in ΔΨ cause mild activation of platelets that remain in circulation, whereas persistent mitochondrial damage leads to depolarization and cell death. This is consistent with the fact that, as demonstrated here, mild hyperpolarization can sustain mitochondrial oxidative phosphorylative function, as well as in studies demonstrating that depolarization is integral in mitochondrial permeability transition pore assembly and phosphatidyl serine externalization,10,48 components of both strongly activated platelets and the apoptotic cascade.
In conclusion, this study demonstrates that SCD patients possess a specific profile of mitochondrial dysfunction and suggests that this alteration in bioenergetics is stimulated by hemolysis and propagates elevated platelet activation. The data herein show that mitochondrial alterations cause platelet activation in vitro and demonstrate the relevance of this pathway in vivo. Collectively, these data advance the understanding of the role of mitochondria in platelet function and suggest that the study of mitochondria in SCD and other hemolytic disease warrants further study.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the Institute of Transfusion Medicine, the Hemophilia Center of Western Pennsylvania, the National Institutes of Health (1R01HL096973), and the American Heart Association (09SDG2150066).
Authorship
Contribution: N.C., C.C., L.G., and S.Z. collected and analyzed data; S.J., S.B., and E.M.N. recruited subjects, obtained written consent, and obtained blood samples; and S.S. and N.C. planned the study, interpreted results, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Sruti Shiva, PhD, Department of Pharmacology & Chemical Biology, Vascular Medicine Institute, BST E1240, University of Pittsburgh, Pittsburgh, PA 15261; e-mail: sss43@pitt.edu.
References
Author notes
N.C. and C.C. contributed equally to this study.