Key Points
P vivax infected cells rosette exclusively to normocytes. Thus, rosetting does not directly facilitate P vivax merozoite invasion.
Glycophorin C (CD236R) mediates vivax malaria parasite rosetting. This finding will help in the search for the P vivax rosette ligand.
Abstract
Rosetting phenomenon has been linked to malaria pathogenesis. Although rosetting occurs in all causes of human malaria, most data on this subject has been derived from Plasmodium falciparum. Here, we investigate the function and factors affecting rosette formation in Plasmodium vivax. To achieve this, we used a range of novel ex vivo protocols to study fresh and cryopreserved P vivax (n = 135) and P falciparum (n = 77) isolates from Thailand. Rosetting is more common in vivax than falciparum malaria, both in terms of incidence in patient samples and percentage of infected erythrocytes forming rosettes. Rosetting to P vivax asexual and sexual stages was evident 20 hours postreticulocyte invasion, reaching a plateau after 30 hours. Host ABO blood group, reticulocyte count, and parasitemia were not correlated with P vivax rosetting. Importantly, mature erythrocytes (normocytes), rather than reticulocytes, preferentially form rosetting complexes, indicating that this process is unlikely to directly facilitate merozoite invasion. Although antibodies against host erythrocyte receptors CD235a and CD35 had no effect, Ag-binding fragment against the BRIC 4 region of CD236R significantly inhibited rosette formation. Rosetting assays using CD236R knockdown normocytes derived from hematopoietic stem cells further supports the role of glycophorin C as a receptor in P vivax rosette formation.
Introduction
In malariology, rosetting is defined by the adherence of uninfected erythrocytes to a Plasmodium spp.-infected erythrocyte. Although the role of rosetting phenomenon remains unknown, 2 major hypotheses have been proposed to explain its importance to the survival and fitness of the malaria parasite. First, the “rosette-assisted invasion” hypothesis, which supposes that rosetting facilitates the encounter of newly emergent merozoites with receptive uninfected red cells bound to the schizont.1-3 Second, that uninfected cells rosetting shield the infected red blood cell (RBC) from the host immune system.2,4
Since its discovery in the 1980s,5,6 rosetting phenomenon has been observed in the 4 major causes of human malaria.1,7-10 However, almost all rosetting studies have focused on P falciparum and its possible role in the pathogenesis of severe disease.11-17 A renewed interest in vivax malaria and a better appreciation of its importance to public health has led to an increased number of studies examining particular aspects of P vivax pathogenesis.18-25 Certainly in the case of P vivax, apart from some initial descriptions on P vivax rosetting8,10 and its association with anemia,26 little has been done to investigate the importance of rosetting to the survival of P vivax within the human host and the molecular mechanisms associated with the formation of rosettes in this species.
Due to the technical challenges associated with P vivax research, the properties, as well as the postulated roles of rosetting in vivax malaria have been extrapolated from experiments conducted on P falciparum.3,4 However, comparisons between these 2 species are problematic, especially when considering the receptor ligand interactions involved in the formation of rosettes. This is because one of the ligands associated with rosette formation in P falciparum (P falciparum erythrocyte membrane protein 1 [PfEMP1]) has no orthologs in P vivax. Thus, all of the receptors on the host red cell corresponding to PfEMP1, such as complement receptor 1 (CD35),27 blood group antigens (A and B),28-30 heparan sulfate,31 and thrombospondin (CD36),32,33 may not be relevant to P vivax rosette formation.
Recent advances in our ability to manipulate ex vivo isolates of P vivax25 have enabled us to conduct detailed investigations on the rosetting phenomenon in this species, allowing us to conduct side-by-side comparisons with rosetting in P falciparum. Specifically, this study has 2 major objectives. First, to evaluate the postulated roles of rosette formation in P vivax, and second, to elucidate erythrocytic receptors that are involved in P vivax rosette formation.
Methods
A summary of the methodology applied, number of isolates used, and corresponding figure index is shown in a flowchart (Figure 1).
Experimental overview. Flowchart showing the summary of methodology applied in this study, and the respective results (figures) are shown in boxes with dotted lines. Note that *P < .05 and **P < .001 indicate these isolates are the same, and used for more than 1 experiment.
Experimental overview. Flowchart showing the summary of methodology applied in this study, and the respective results (figures) are shown in boxes with dotted lines. Note that *P < .05 and **P < .001 indicate these isolates are the same, and used for more than 1 experiment.
Blood sample collection
A total of 87 fresh isolates of P vivax and 77 fresh isolates of P falciparum were used in this study. Another 48 cryopreserved P vivax isolates were also used. All isolates were obtained from malaria patients presenting at the clinics of the Shoklo Malaria Research Unit (SMRU) in northwestern Thailand. The clinical samples were collected and tested in accordance with protocols approved by The Center for Clinical Vaccinology and Tropical Medicine at University of Oxford (OXTREC 58-09 and OXTREC 04-10), in consultation with the Ethics Committee of the Faculty of Tropical Medicine at Mahidol University. The study was conducted in accordance with the Declaration of Helsinki. Blood samples were collected using BD Vacutainer with lithium heparin anticoagulant. ABO blood group of each sample was determined via standard hemagglutination with TransClone anti-A and anti-B antibodies (Bio-Rad, Hercules, CA). A thick and thin blood smear was prepared from each blood sample to determine the species of malaria parasites involved, parasitemia, and the predominant erythrocytic stage of the parasite. Reticulocyte concentrations were prepared from human cord blood using the method outlined by Russell et al.24
Rosetting assay on fresh samples
Plasmodium sp. infected blood samples with at least 70% of parasite population in “ring” forms were cultivated at 3% hematocrit using McCoy’s 5A medium enriched with 20% homologous serum, using the method described by Russell et al.24 Samples were checked frequently, and sampled at ring, early trophozoite, late trophozoite, and schizont stages. The presence of rosettes and living parasites were detected and quantified using a novel Giemsa subvital staining methodology,34 modified from techniques applied in a previous study.8 Briefly, the sampled culture suspension was stained with Giemsa (the final stain concentration was 5%) for 15 minutes. A small volume of this suspension (7.5 μl) was used to make a wet mount with 22 × 32 mm (0.17 mm thickness) glass cover slip. The wet mount was examined immediately with light microscope under oil immersion magnification. Rosetting rate was then determined by examining 200 infected erythrocytes (in McCoy’s 5A medium enriched with 20% homologous serum).
Rosetting assay on cryopreserved samples
Vivax malaria blood samples with at least 70% of parasite population in “ring” forms were cryopreserved using the glycerolyte method as described previously.22,35 Prior to cryopreservation, vivax malaria samples with parasitemia lower than 0.1% were subjected to 75% Percoll density gradient centrifugation to concentrate the parasitized erythrocytes. The cryopreserved samples were then thawed using the sodium chloride method.36 The thawed samples were then matured, and rosetting assay was conducted as described above. Rosetting prevalence of each cryopreserved isolate was determined.
Erythrocyte subset preference study
Blood samples infected with either P vivax or P falciparum were cultivated and matured until at least 60% of the parasite population reached schizogony. One hundred µL of each culture suspension was used as a control where its rosetting rate was determined. Matured parasites from the remaining culture suspension were then concentrated with magnetic activated cell sorting column (Miltenyi Biotec). The concentrated infected packed erythrocytes (volume = ×) were then mixed with 0.5 × volume of uninfected packed normocytes, and 0.5 × volume of uninfected reticulocytes. The cell mixtures were suspended in enriched McCoy’s 5A medium as described above and mixed well by vortexing to disrupt rosettes. Importantly, in a preliminary study (data not shown), we found that rosettes were disrupted even after 1 minute of vortexing (we used 2 minutes to be absolutely sure of total disruption). The free infected RBCs (IRBCs) started reforming rosettes within seconds, and the vortexed isolate returned to the original rosetting frequency within 5 minutes. To ensure maximal rosette formation, we incubated the vortexed blood mixtures at 37°C for 30 minutes. Thereafter, rosetting assay was conducted as described above. Types of erythrocytes involved in each rosette were studied, and the erythrocyte subset preference for rosetting in P vivax and P falciparum were then determined.
Erythrocyte receptor-blocking study using Ag-binding fragments and antibodies
Preliminary trials clearly showed that the intact anti-glycophorin C and A antibodies caused considerable hemagglutination (not observed with CD35 antibodies). Therefore, it was necessary to use Ag-binding (FAB) fragments fragments of anti-CD236R and CD235a monoclonal antibodies.
Mouse anti-human CD236R clone BRIC 4 IgG (Thermo Scientific Pierce), mouse anti-human CD236R clone BRIC 10 IgG (Abcam, Cambridge, United Kingdom), and mouse anti-human glycophorin A IgG (Abcam) were used for this experiment. Fab fragments of the respective antibodies were prepared using the Pierce Fab Micro Preparation Kit and Resin Kit (Thermo Scientific Pierce). Plasmodium sp. infected blood samples were cultured and matured as described above. Then, the culture suspensions were vortexed vigorously for 2 minutes to mechanically dissociate any preformed rosettes. Each culture suspension was divided into four 1.5 mL microcentrifuge tubes, where each tube of culture suspension was added with one type of the prepared antibody or Fab fragments (50 μg/mL final concentration), and one treatment-free tube was the negative control for the experiment. The mixtures were incubated for 30 minutes at 37°C. Finally, rosetting assay was conducted on each tube of suspension as described above. In addition to the Fab treatments, we also used mouse anti-human CD35 antibody (BD Pharmigen) as a positive control.
Hematopoietic cell culture and CD236R knockdown experiments
Cryopreserved human umbilical cord blood mononuclear cells were expanded using a method described by Giarratana et al37 with few modifications. Briefly, the CD34+ hematopoietic progenitors were isolated by immunomagnetic selection using CD34 MicroBead Kit (Miltenyi Biotec, Singapore). The CD34+ cells were harvested during 3 weeks in IMDM GlutaMAX (Gibco), supplemented with 330 μg/mL of human holotransferin (Sigma-Aldrich, Singapore), 10 μg/mL recombinant human insulin (SAFC Biosciences), 2 IU/mL heparin (Sigma-Aldrich), and 5% AB blood group serum. Between days 0 and 7, the following additional supplementation were incorporated: 10−6 M of hydrocortisone (Stemcell Technologies), 100 ng/mL of stem cell factor (PeproTech), 5 ng/mL of IL-3 (PeproTech), and 3 UI/mL of erythropoietin (Epo) (Stemcell Technologies); between days 7 and 11, 100 ng/mL of stem cell factor and 3 UI/mL of Epo; and after day 11 only Epo was added.
One day after the immunomagnetic sorting, the CD34+ cells were transduced (MOI of 5) with shRNA (short hairpin RNA) lentivector MISSION, shRNA Target Set NM_002101 (Glycophorin C [CD236R]) (Sigma-Aldrich) in the presence of 8 μg/mL of Polybrene (Sigma-Aldrich). Fifteen days after the transduction, green fluorescent protein (GFP) positive and negative cells were sorted using BD Influx Flow Cytometer (BD Biosciences). The day after, GFP positive and negative fractions were incubated with P vivax late stages isolated by magnetic sorting as described previously.38 The level of CD236R expression in each subset was measured by flow cytometry using anti-human CD236R, clone BRIC 4 (Thermo Scientific Pierce) antibody as primary staining, and anti-mouse e660 (eBioscience) as secondary staining. The cells were acquired on LSRII Flow Cytometer (BD Biosciences) and analyzed with FlowJo software (Tree Star).
Statistical analyses
In most cases, the rosetting rate was defined as the percentage of 200 infected erythrocytes that formed stable adhesion with at least 1 uninfected erythrocyte after 15 minutes of postvortex incubation (as observed under 100× oil immersion). A giant rosette is defined as a rosetting complex with the participation of at least 10 uninfected erythrocytes.9-11 As most of the data were not normally distributed, nonparametric analysis such as Mann-Whitney U, Kruskal-Wallis (more than 2 groups), and Friedman tests (repeated measures) were used. In the latter 2 tests, Dunn post hoc analysis was used. All statistical analysis used Prism 5 for Windows (version 5.01), Software MacKiev.
Results
Cryopreservation and rosetting
At the beginning of this study, for purposes of practicality, we used cryopreserved isolates of P vivax. However, it soon became evident that the rates of rosetting in these cryopreserved-then-thawed samples were considerably lower than those previously reported from fresh isolates.10 Subsequently, we decided to focus our work on fresh isolates rather than cryopreserved (with the exception of the knockdown experiment). Certainly, when we compared our data on rosetting prevalence from fresh isolates with cryopreserved isolates, we showed that cryopreservation was associated with a lower incidence of rosette formation; whereas the median rosetting rate for fresh isolates was 23.80% (interquartile range [10.00% to 31.00%]), the median rosetting rate for cryopreserved isolates was 0.00% (interquartile range [0.00% to 14.85%]) on P (2-tailed) < .0001, Mann-Whitney U test (see supplemental Figure 1 on the Blood Web site).
Rosetting properties of P vivax
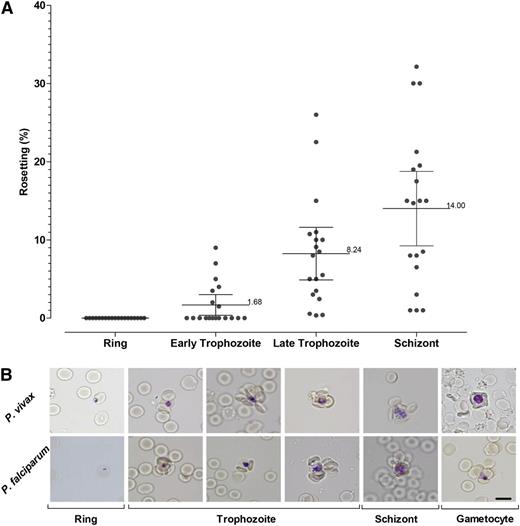
Rosette formation of P vivax increased with the parasite erythrocytic maturation (Figure 2A). In this study, no early stage (ring form) was found to be involved in rosette formation. Rosette formation of P vivax was noted 24 hours postcultivation, represented by early trophozoite stage. The rosetting rate increased markedly between early trophozoite stage and the late trophozoite stage. After 30 hours, the rosetting development continued, albeit at a much lower rate, until the parasite reached schizogony at hour 44.
Rosetting kinetics. (A) Plot showing the kinetics of rosetting development in 47 P vivax isolates matured ex vivo. (B) Representative images of rosettes formed by different stages of P vivax and P falciparum are shown after Giemsa subvital staining process.34
Rosetting kinetics. (A) Plot showing the kinetics of rosetting development in 47 P vivax isolates matured ex vivo. (B) Representative images of rosettes formed by different stages of P vivax and P falciparum are shown after Giemsa subvital staining process.34
Giant rosettes were found occasionally in P vivax isolates; 10% of the rosettes found in P vivax isolates were giant rosettes. On the other hand, only 0.5% of the rosettes found in P falciparum isolates were giant rosettes. Besides the asexual stages, gametocytes of P vivax were found to be involved in rosette formation (Figure 2B). Involvement of gametocyte-infected erythrocytes in rosette formation was found in P falciparum isolates as well (Figure 2B).
Rosetting rate and rosetting prevalence
Rosetting occurs as frequently in isolates of P vivax (91.5%; 43 of 47) as it does in P falciparum (79.5%; 31 of 39) confirming earlier studies by Udomsanpetch et al.10 Interestingly, the median rate of rosetting was significantly higher in P vivax (24.5%) when compared with P falciparum (9.0%) isolates from the same area in Thailand (P < .001) (Figure 3A). No significant correlation was found between the rosetting rate and parasitemia or ABO blood groups in both vivax and falciparum malaria patients (Figure 3B-C). The parasitemia range for P vivax and P falciparum was 0.01% to 1.70% and 0.10% to 11.00%, respectively.
Factors affecting rosetting formation in P vivax and P falciparum. (A) Comparison of rosetting rate between P vivax and P falciparum isolates from the Thailand-Myanmar border. The median percentage of P vivax IRBCs is significantly higher than P falciparum; P (2-tailed) < .001. (B) Rosetting rate of P vivax and P. falciparum isolates found in different human ABO blood groups. No significant association was found between ABO phenotype of the malaria and occurrence of P vivax (P = .28) or P falciparum (P = .20). (C) Plot of rosetting rate of P vivax and P falciparum isolates against the original parasitemia of malaria patients presenting for treatment. No significant correlation was observed between patient parasitemia and rosetting for P vivax (Spearman r = −0.10; 95% CI [−0.39 to 0.20]; P = .50) or P falciparum (Spearman r = −0.06; 95% CI [−0.38 to 0.27]; P = .71). (D) Rosetting rate of P vivax and P falciparum isolates against peripheral reticulocyte counts in malaria patients. No significant correlation was observed between rosetting and patient reticulocyte counts for either P vivax (Spearman r = 0.33; 95% CI [−0.04 to 0.62]; P [2-tailed] = .07) or P falciparum (Spearman r = −0.13; 95% CI [−0.49 to 0.28]; P = .52).
Factors affecting rosetting formation in P vivax and P falciparum. (A) Comparison of rosetting rate between P vivax and P falciparum isolates from the Thailand-Myanmar border. The median percentage of P vivax IRBCs is significantly higher than P falciparum; P (2-tailed) < .001. (B) Rosetting rate of P vivax and P. falciparum isolates found in different human ABO blood groups. No significant association was found between ABO phenotype of the malaria and occurrence of P vivax (P = .28) or P falciparum (P = .20). (C) Plot of rosetting rate of P vivax and P falciparum isolates against the original parasitemia of malaria patients presenting for treatment. No significant correlation was observed between patient parasitemia and rosetting for P vivax (Spearman r = −0.10; 95% CI [−0.39 to 0.20]; P = .50) or P falciparum (Spearman r = −0.06; 95% CI [−0.38 to 0.27]; P = .71). (D) Rosetting rate of P vivax and P falciparum isolates against peripheral reticulocyte counts in malaria patients. No significant correlation was observed between rosetting and patient reticulocyte counts for either P vivax (Spearman r = 0.33; 95% CI [−0.04 to 0.62]; P [2-tailed] = .07) or P falciparum (Spearman r = −0.13; 95% CI [−0.49 to 0.28]; P = .52).
Erythrocyte subset preference study
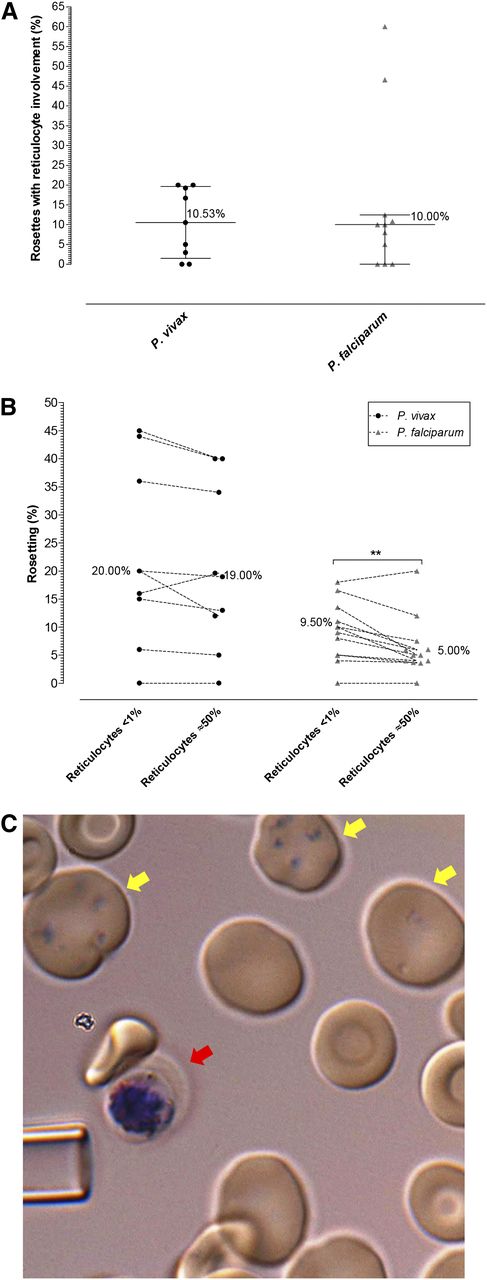
The availability of reticulocytes in the peripheral blood of vivax or falciparum malaria patients had no bearing on the incidence of rosettes (Figure 3D). Close inspection of rosettes from 9 vivax malaria (562 rosettes) and 11 falciparum (391 rosettes) malaria isolates revealed that only ∼10% of rosettes involved attachment of reticulocytes (and in these cases only 1 reticulocyte per rosette could be observed); the majority of rosettes were exclusively composed of mature erythrocytes (Figure 4A).
P vivax rosetting preference. (A) Percentage of rosettes associated with at least 1 reticulocyte in P vivax and P falciparum isolates in patient isolates already containing late-stage parasites (no ex vivo maturation). (B) Differences in rosetting rate of P vivax and P falciparum isolates in environment with <1% reticulocytes and approximately 50% reticulocytes (achieved by concentrating host reticulocytes on a 75% Percoll gradient). Lines connect paired observations. Altering the reticulocyte concentration had little effect on P vivax rosette formation; however, the number of normocytes attached to the P vivax IRBC was notably reduced in the treatment of enriched reticulocytes. Interestingly, an increase in the available reticulocytes reduced the ability of P falciparum to rosette (P < .01). (C) Reticulocytes were rarely associated with rosettes irrespective of the group or species observed. Here, a single normocyte is rosetting on a P vivax schizont (red arrow). The yellow arrows point to Heilmeyer stage IV reticulocytes subvitally stained with Giemsa. The tip of a glass micropipette with an internal diameter of 6 um is shown for scale.
P vivax rosetting preference. (A) Percentage of rosettes associated with at least 1 reticulocyte in P vivax and P falciparum isolates in patient isolates already containing late-stage parasites (no ex vivo maturation). (B) Differences in rosetting rate of P vivax and P falciparum isolates in environment with <1% reticulocytes and approximately 50% reticulocytes (achieved by concentrating host reticulocytes on a 75% Percoll gradient). Lines connect paired observations. Altering the reticulocyte concentration had little effect on P vivax rosette formation; however, the number of normocytes attached to the P vivax IRBC was notably reduced in the treatment of enriched reticulocytes. Interestingly, an increase in the available reticulocytes reduced the ability of P falciparum to rosette (P < .01). (C) Reticulocytes were rarely associated with rosettes irrespective of the group or species observed. Here, a single normocyte is rosetting on a P vivax schizont (red arrow). The yellow arrows point to Heilmeyer stage IV reticulocytes subvitally stained with Giemsa. The tip of a glass micropipette with an internal diameter of 6 um is shown for scale.
As reticulocytes only make up a small portion of the peripheral blood (<2%), we wanted to ensure that the lack of reticulocyte involvement in rosette formation was not only a function of the lower probability of parasitized cells encountering the reticulocyte (rare) versus the commonly found normocyte. To test this, we added magnetic activated cell sorting column-concentrated P vivax and P falciparum IRBCs to blood samples containing enriched reticulocytes (50% +/−10). When the reticulocyte-enriched experiment group was compared with the paired rosetting data from the control group (the nonenriched blood) using Wilcoxon Signed-Rank test, rosetting rates for P vivax was unaffected, whereas P falciparum rosetting rates actually decreased in the enriched reticulocyte treatments P < .01 (1-tailed, Mann-Whitney U test) (Figure 4B-C). Although we did observe the occasional P vivax rosette with reticulocytes attached in the enriched treatment, the formation of rosettes almost exclusively involved normocytes, despite the even chance to encounter reticulocytes. Since there may be concerns regarding the difference between cord blood reticulocytes and adult reticulocytes, we repeated the reticulocyte enrichment experiment using 3 more fresh isolates of P vivax and reticulocytes isolated from 2 adult donors. We found no significant difference between the adult and cord-blood–derived reticulocytes in their lack of involvement in P vivax rosette formation (supplemental Figure 2).
Rosetting inhibition assay
Fab and antibody blocking experiment
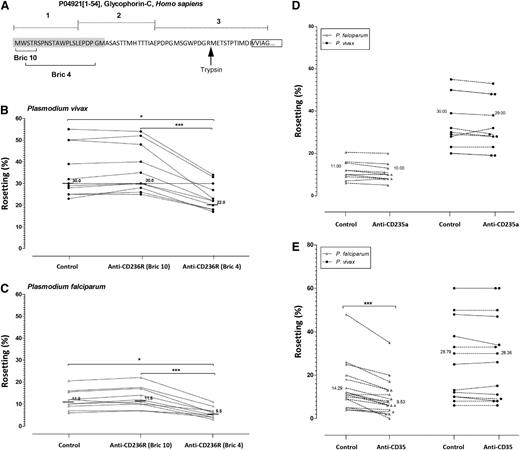
In vivax malaria isolates tested, Fab of mouse anti-human CD236R IgG (clone BRIC 4) (Figure 5A shows where this Fab clone binds) significantly reduced rosetting rate by 27% (P < .05; Friedman analysis with the Dunn multiple comparison test) (Figure 5B). Interestingly, BRIC 4 Fabs reduced rosette formation in falciparum malaria isolates by at least half (Figure 5C). Importantly, the Fab fragment of another anti-CD236R antibody had no effect on rosette formation in the same paired experiments demonstrating the specificity of the inhibition of BRIC 4 antibodies (Figure 5B). Anti-glycophorin A Fab fragments had no effect on rosette formation for both species (Figure 5D).
Antibody blocking rosette formation. (A) Schematic diagram of the target sites of anti-CD236R antibody clone BRIC 10 and anti-CD236R antibody clone BRIC 4 on human CD236R structure. The target site of trypsin on this sialoglycoprotein is shown by the black arrow. (B-C) Rosetting inhibition in P vivax and P falciparum caused by Fab fragments specifically targeting the BRIC 10 and BRIC 4 locations on CD236R. BRIC 4 showed a significant reduction in rosetting of P falciparum isolates (P < .0001) and P vivax isolates (P < .0001) studied. A paired Student t test was conducted. (D) Rosetting inhibition by mouse anti-human CD35 antibody. Unlike in P vivax, this antibody significantly reduced rosetting rate of P falciparum isolates tested (P < .0001). (E) Comparison of rosetting rates between the control and cells incubated with Fab fragments of mouse anti-human glycophorin A antibody from P falciparum and Pvivax isolates recruited. There was no significant difference between the control group and the “anti-glycophorin A” group in P vivax and P falciparum isolates studied.
Antibody blocking rosette formation. (A) Schematic diagram of the target sites of anti-CD236R antibody clone BRIC 10 and anti-CD236R antibody clone BRIC 4 on human CD236R structure. The target site of trypsin on this sialoglycoprotein is shown by the black arrow. (B-C) Rosetting inhibition in P vivax and P falciparum caused by Fab fragments specifically targeting the BRIC 10 and BRIC 4 locations on CD236R. BRIC 4 showed a significant reduction in rosetting of P falciparum isolates (P < .0001) and P vivax isolates (P < .0001) studied. A paired Student t test was conducted. (D) Rosetting inhibition by mouse anti-human CD35 antibody. Unlike in P vivax, this antibody significantly reduced rosetting rate of P falciparum isolates tested (P < .0001). (E) Comparison of rosetting rates between the control and cells incubated with Fab fragments of mouse anti-human glycophorin A antibody from P falciparum and Pvivax isolates recruited. There was no significant difference between the control group and the “anti-glycophorin A” group in P vivax and P falciparum isolates studied.
CD35 blocking experiment
Complement receptor 1 or CD35 is 1 of the erythrocytic receptors involved in rosetting of P falciparum.27 Certainly in the P falciparum isolates we tested, the rosetting rate was significantly inhibited (33.3%) by the mouse anti-human CD35 antibody (P < .0001, R = 0.8236; 95% CI [3.026 to 6.504]). However, CD35 had no effect on rosette formation in the P vivax isolates tested (Figure 5E).
Hematopoietic cell culture and CD236R knockdown
Although the above Fab-mediated inhibition assays provide useful clues to the identity of the host rosetting receptor, such an approach is limited by potential off-target effects (such as steric hindrance or changes to membrane rigidity).39 To limit these unwanted “off-target effects,” we employed a transgenic method to knockdown the expression of CD236R to produce normocytes mostly deficient in this receptor (Figure 6A). Phenotyping of GF- positive and GFP-negative cells (according to BRIC 4 region recognition [Figure 5A]) showed a knockdown of 81.5% ± 2.5% in CD236R expression (Figure 6B). It should be noted that the method we used to knockdown the expression of glycophorin A was different to the one recently used by Bei et al40 (where puromycin and neomycin rather than GFP was used for selection) but with the same knockdown efficiency. It is important to note that the erythrocytes generated from the CD236R knockdown had a normal phenotype in terms of 7 other characteristic erythrocytic receptors (supplemental Figure 3).
Transgenic approach to investigate the role of CD236R in P vivax rosetting. (A) Experimental design of schizont P vivax rosetting assay with cultured RBCs (cRBCs) generated from CD34+ hematopoietic stem cells. The stable knockdown of CD236R (glycophorin C) is obtained using Sigma lentivector with GFP and shRNA against CD236R cassette expression. The 2 subsets of cells: CD236R knockdown and CD236R+ cells were separated by flow cytometry using GFP expression, 1 day before performing the rosetting assay with P vivax schizonts isolated by magnetic sorting. (B) Flow cytometry histograms showing CD236R expression in GFP-positive cells (CD236R knockdown cells [green line]), GFP-negative (CD236R+ cells [black line]), and unstained cells (gray line). (C) Plots showing schizont P vivax rosetting with cRBC with CD236R knockdown (CD236R KD) and without knockdown (CD236+) with bright field (BF), Hoechst, and GFP detection. (D) Frequency of schizonts with or without rosetting in the presence of CD236R knockdown cRBCs or of CD236+ cRBCs showing a significant difference in proportion of schizonts able to form rosettes between the 2 different types of cRBCs (CD236R KD and CD236+).
Transgenic approach to investigate the role of CD236R in P vivax rosetting. (A) Experimental design of schizont P vivax rosetting assay with cultured RBCs (cRBCs) generated from CD34+ hematopoietic stem cells. The stable knockdown of CD236R (glycophorin C) is obtained using Sigma lentivector with GFP and shRNA against CD236R cassette expression. The 2 subsets of cells: CD236R knockdown and CD236R+ cells were separated by flow cytometry using GFP expression, 1 day before performing the rosetting assay with P vivax schizonts isolated by magnetic sorting. (B) Flow cytometry histograms showing CD236R expression in GFP-positive cells (CD236R knockdown cells [green line]), GFP-negative (CD236R+ cells [black line]), and unstained cells (gray line). (C) Plots showing schizont P vivax rosetting with cRBC with CD236R knockdown (CD236R KD) and without knockdown (CD236+) with bright field (BF), Hoechst, and GFP detection. (D) Frequency of schizonts with or without rosetting in the presence of CD236R knockdown cRBCs or of CD236+ cRBCs showing a significant difference in proportion of schizonts able to form rosettes between the 2 different types of cRBCs (CD236R KD and CD236+).
Initially, this work needed to be conducted in Singapore rather than in Mae Sod; therefore, we had to rely on cryopreserved isolates of P vivax to conduct this experiment. Of the 3 P vivax cryopreserved isolates used, only one showed very high levels of rosetting (>50%). Despite this limitation, differences in rosetting between the control normocytes (CD236R positive) and CD236R knockdown normocytes was immediately evident. After examination of 200 fields (×1000) and counting 50 P vivax IRBCs per treatment, only 4 of 50 were rosetting with CD236R knockdown erythrocytes compared with 27 of 50 in the control (χ-square = 22.6, d.f. = 1, P < .0001 [χ-square with Yates correction]). Because we were concerned about possible confounders associated with the use of cryopreserved isolates (supplemental Figure 1), we repeated this experiment, this time using 3 fresh isolates. As per the original experiment, we found that the CD236R knockdown erythrocytes significantly reduced the formation of rosettes (P = .007) (Figure 6C-D). It is important to understand that although the cells were sorted on GFP, this protein has about 26 hours half-life.41 Therefore, in mammalian cells, the signal gradually diminishes as the erythrocyte matures. Consequently, the normoblasts (Heilmeyer stage 0) and early reticulocytes (Heilmeyer stages I, II, and III) have bright signals. Mature reticulocytes (Heilmeyer stage IV) and normocytes have little or no discernable signal (Figure 6C). An unintended benefit of this age-related GFP signal output was that in the 4 rosettes observed in the CD236R knockdown, no rosette was associated with GFP-positive cells (reticulocytes), thus further supporting our data on P vivax rosette normocytic preference.
Discussion
The biological role of P vivax and P falciparum rosetting
Although the role of rosetting in the pathogenesis of malaria remains controversial, the real mystery surrounding this phenomenon is what advantage it brings to the intra-erythrocytic parasite. While not providing a definitive answer to this question, our data provides compelling evidence that rosetting does not facilitate the invasion of merozoites. We have 2 lines of evidence disputing the “rosette-assisted invasion” hypothesis.
Foremost and most convincingly, our data shows that P vivax preferentially forms rosettes with mature red cells and not reticulocytes. If P vivax rosettes were to truly assist in merozoite invasion, one would hypothesize that its schizonts would preferentially adhere to uninfected reticulocytes; thereby improving invasion success by the provision of immediate access to proximal reticulocytes (which are generally scarce in the peripheral circulation). However, the observations of naturally occurring rosettes from fresh isolates and our reticulocyte enrichment experiment clearly show that rosettes favor normocytes rather than reticulocyte attachment. As P vivax merozoites exclusively invade reticulocytes, there would be little advantage for the parasite species to have its schizonts binding with normocytes, which are not receptive to invasion. As P falciparum merozoites can invade both reticulocytes and normocytes, its preferential rosette formation with normocytes does not discount the possibility that rosetting may improve the invasion success of merozoites in this species. However, convincing data from Clough et al shows that rosette-forming strains of P falciparum have no invasion advantage over nonrosetting strains.42 Unpublished data (supplemental Figure 4) derived from an earlier study on P vivax invasion,24 clearly shows that the rate of rosetting within an isolate bears no correlation with invasion success.
A second line of evidence against the “rosette-assisted invasion” hypothesis is that P vivax and P falciparum start rosetting at early trophozoite stage, at least 24 hours prior to the expulsion of merozoites from the segmenting schizont. Whereas some may suggest that the developing parasite is getting an “early start” on the collection of receptive cells, such an effort would be wasted as the shear forces present in the host circulation, and physical barriers such as the splenic red pulp would undoubtedly disrupt the rosette numerous times within the 24-hour time interval between the initiation of rosette formation and schizont rupture. Perhaps even more convincing is our observation that P vivax and P falciparum gametocytes also form rosettes, a new finding contrary to the earlier report from Lowe et al.1
Clearly, data from this and earlier studies24,42 suggest that rosetting does not directly assist in the targeting or invasion of malaria parasites into uninfected host erythrocytes. Although we do not have data to support the alternative hypothesis, which proposes that rosetting shields the iRBC from the host immune system (first postulated by Wahlgren et al),3 we agree that this is the most likely reason for such an adaptation in Plasmodium spp. It certainly seems intuitive that iRBC containing mature stages (and the associated plethora of parasite antigens expressed on the red cell membrane) would greatly benefit from a cloak of host RBCs. Again, this hypothesis is relatively speculative, and we hope that future studies will focus on the role of rosetting in the protection of Plasmodium spp. from the host immune system.
The observation that rosetting occurs as frequently in P vivax as it does in P falciparum, raises a number of important questions regarding the clinical significance of rosetting in vivax malaria. Although our data are from de-identified samples (thus not allowing direct clinical correlates with the rosetting rate), we do know that all our samples were collected from uncomplicated cases of vivax malaria. As such, it is difficult to speculate on the direct role of rosetting on P vivax pathogenesis. We certainly hope that future clinical investigations into severe vivax malaria includes rosetting as an important parasitologic parameter.
Erythrocyte receptors mediating rosetting in fresh isolates of P vivax
Whereas numerous studies have shown that the human ABO blood group and CR1 (CD35) are key determinants on P falciparum rosetting,7,27-30 this does not seem to be the case with P vivax. The absence of association between P vivax rosetting and the ABO blood group agrees with the findings from an earlier study by Chotivanich et al.8 The lack of association between the ABO blood group and CR1 in P vivax should not be surprising as the ligand responsible for this interaction in P falciparum (PfEMP1) is absent from P vivax. Our efforts to shortlist novel receptors mediating rosetting in P vivax were aided by unpublished data from one of the authors of this study, which indicated that CD236R modulates P falciparum rosetting in a PfEMP1-independent manner. Certainly, our results show that specifically blocking the BRIC 4 region (amino acid residues 2-21) of CD236R significantly inhibits rosette formation in P vivax and P falciparum. As most of the BRIC 4 region is located in segment 1 of CD236R, it is unlikely that the Gerbich and Yus mutations (which cause major deletions to regions 2 and 3) will have any effect on its ability to form rosettes (Figure 5A). Although the Leach mutation would indeed remove the residues of interest, we did not attempt to source erythrocytes from this rare phenotype, as the mutant cells are elliptical (not biconcave) in shape, and have a profoundly modified biomechanical profile that would confound any conclusions regarding their ability to rosette (irrespective of the complete absence of CD236R in the Leach-type cells). It is interesting to note that trypsin treatment of erythrocytes completely removes 2 extracellular domains of CD236R (1 and 2) (Figure 5A), resulting in the complete abrogation of rosetting in P vivax.24
To better understand the importance of CD236R as a key host receptor for P vivax rosetting, we knocked down its expression in normocytes derived from hematopoietic stem cells. Rosetting assays using these CD236R knockdown erythrocytes clearly show the significant reduction of rosetting phenomena in P vivax compared with normocytes (from the same culture) expressing this receptor.
One problem with the idea that “CD236R is important to rosetting,” is that the presentation of this receptor decreases during reticulocyte maturation to normocyte; however, it is still highly expressed in both subsets.43 As P vivax does not rosette well with reticulocytes, one has to question as to what prevents the interaction between reticulocyte CD236R and the yet to be discovered P vivax rosetting ligands. We postulate that the rigid biomechanical properties of reticulocytes (reticulocytes are significantly stiffer than normocytes),43 inhibit the formation of close bonds with the P vivax IRBC. Close examination of images of P vivax rosettes, especially in Figure 4C, show the tight association of the normocyte and infected cell (ie, the flat face of the biconcave disk to the IRBC). The globular and stiff reticulocyte inhibits intimate contact with the P vivax IRBC. Certainly, our efforts to manipulate reticulocytes into direct contact with P vivax cells (using a micropipette) failed to promote rosetting.
Conclusion
Rosetting is a common property of mature intra-erythrocytic malaria parasites, occurring as frequently in P vivax as it does in P falciparum. The high frequency of rosetting in patient isolates suggests that it provides P vivax with a survival advantage. Our ex vivo experiments indicate that in the case of P vivax, this advantage is most likely to be associated with immune invasion rather than facilitating the invasion of reticulocytes. Although ligands and receptors primarily involved in the formation of rosettes in P vivax still remain a mystery, we provide strong evidence that CD236R is involved, whereas receptors traditionally involved in P falciparum rosettes, such as ABO antigens and CD35, are not. We also wish to stress that as with P falciparum, there is likely to be more than 1 host receptor involved in P vivax rosetting. Future work should focus on the discovery of P vivax ligands that modulate the attachment of normocytic CD236R and other possible receptors involved in the rosetting process.
Presented in part at the International Malaria Symposium, Sabah, Malaysia, April 16-17, 2013, and the Gordon Research Conference (Malaria), Lucca, Italy, August 4-9, 2013.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the staff of SMRU who assisted in the management of this research study. B.R. and L.R are adjunct Professors at the Department of Parasitology, University of Malaya, Kuala Lumpur, Malaysia. We would like to thank the SIgN flow cytometry core lead by Dr Anis Larbi and his team members, Ivy Low Nurhidaya Shadan, Seri Mustafahi, and Nurhidaya Shadan.
This work was supported by the University of Malaya Research Grant (PV044-2012-A) (W.C.L.), University of Malaya, High Impact Research Fund UM-MOHE (UM.C/625/1/HIR/MOHE/CHAN/14/3), the Ministry of Higher Education Malaysia (Y.L.L. and M.Y.F.), the SIgN and the Horizontal Programme on Infectious Diseases (L.R.), and a Young Investigator Grant (BMRC YIG Grant No:13/1/16/YA/009) under the Agency for Science, Technology, and Research (Singapore) (B.M.). SMRU is part of the Mahidol Oxford University Research Unit, supported by The Wellcome Trust of Great Britain. And additional funding was provided by the National University of Singapore Faculty Start-Up Grant and the Singapore National Medical Research Council (NMRC/CBRG/0047/2013) (B.R. and B.M.).
Authorship
Contribution: W.C.L., B.R., B.M., M.M., R.Z., R.S., J.S.C., L.A., and F.T.M.C. carried out laboratory work, collected, and analyzed the data; R.M., F.N., Y.L.L., and M.Y.F. performed the clinical management of patients, ethical clearance, and collection and processing of the blood samples; W.C.L., B.R., P.P., Y.L.L., M.Y.F., B.M., and L.R. participated in data interpretation and helped to draft the manuscript; and all authors read and approved the final manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Bruce Russell, Vivax Malaria Laboratory, Department of Microbiology, Yong Loo Lin School of Medicine, National University of Singapore, 5 Science Dr 2, Blk MD4, Level 3, Singapore 117597, Singapore; e-mail: micbmr@nus.edu.sg.

![Figure 3. Factors affecting rosetting formation in P vivax and P falciparum. (A) Comparison of rosetting rate between P vivax and P falciparum isolates from the Thailand-Myanmar border. The median percentage of P vivax IRBCs is significantly higher than P falciparum; P (2-tailed) < .001. (B) Rosetting rate of P vivax and P. falciparum isolates found in different human ABO blood groups. No significant association was found between ABO phenotype of the malaria and occurrence of P vivax (P = .28) or P falciparum (P = .20). (C) Plot of rosetting rate of P vivax and P falciparum isolates against the original parasitemia of malaria patients presenting for treatment. No significant correlation was observed between patient parasitemia and rosetting for P vivax (Spearman r = −0.10; 95% CI [−0.39 to 0.20]; P = .50) or P falciparum (Spearman r = −0.06; 95% CI [−0.38 to 0.27]; P = .71). (D) Rosetting rate of P vivax and P falciparum isolates against peripheral reticulocyte counts in malaria patients. No significant correlation was observed between rosetting and patient reticulocyte counts for either P vivax (Spearman r = 0.33; 95% CI [−0.04 to 0.62]; P [2-tailed] = .07) or P falciparum (Spearman r = −0.13; 95% CI [−0.49 to 0.28]; P = .52).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/123/18/10.1182_blood-2013-12-541698/4/m_e100f3.jpeg?Expires=1769141221&Signature=ZnBmSmOzYLdiV~1IdBUiyMxvNBsUlNC08RF4Z4uYlvXV6MakToGKhk5cmYuPXsl2nonLG7irsWaSG15DKkE9WTVUhUw1XPTTXKHOhteHM4vmcbr7C2gW3QWtK05B56Utj8uumpXs0LdbzyAe4O4bvBST-DtetxK64edXMaG5aG29ZR~tSHCJvda02LUl5R-on7b0meKUPsKPy0aAlNnhcrvowUtYNsJt1ZBz0is61Xd49C-7AQuKFeRHl63dSS0SEARx-ymUHIsKMqBn1TH1pB2cBBcjApr4NbtTay-wipqngrXRfgX4lc~~M6i7cgvfZ4ATe-DIZaIFn~1ZZqeQ1A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 6. Transgenic approach to investigate the role of CD236R in P vivax rosetting. (A) Experimental design of schizont P vivax rosetting assay with cultured RBCs (cRBCs) generated from CD34+ hematopoietic stem cells. The stable knockdown of CD236R (glycophorin C) is obtained using Sigma lentivector with GFP and shRNA against CD236R cassette expression. The 2 subsets of cells: CD236R knockdown and CD236R+ cells were separated by flow cytometry using GFP expression, 1 day before performing the rosetting assay with P vivax schizonts isolated by magnetic sorting. (B) Flow cytometry histograms showing CD236R expression in GFP-positive cells (CD236R knockdown cells [green line]), GFP-negative (CD236R+ cells [black line]), and unstained cells (gray line). (C) Plots showing schizont P vivax rosetting with cRBC with CD236R knockdown (CD236R KD) and without knockdown (CD236+) with bright field (BF), Hoechst, and GFP detection. (D) Frequency of schizonts with or without rosetting in the presence of CD236R knockdown cRBCs or of CD236+ cRBCs showing a significant difference in proportion of schizonts able to form rosettes between the 2 different types of cRBCs (CD236R KD and CD236+).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/123/18/10.1182_blood-2013-12-541698/4/m_e100f6.jpeg?Expires=1769141221&Signature=hVAAPvHyw-y6U5cNftqylo6nfTN9dtrAKSYS22~dbZqq~qNZ2IQSVqUrjW-6x~lNFVUS1CqqgeKZOI-pGSpa8iEmeoWfWCcDJAWpXVFx9lX2Adq05v4AuA-aS21UyVTnJ~VdtXK3V5axOlmKuSBd2RfbjqmwdIA~BWKKkKegizCAybpD~Df7HgQSrANIi-wgCK-l4teWLiPGaknuAdQKgnxNGHctj-F32~1ImE~MOq0v2H0mYC13P7HiTid8pKrA9vDgHv9gG4nVC3aRIbEIvQJz4Eom1yvFYkh0MjSiLrtPWByO1Egxk~1zRo0z9I6GdoeOshlxuylogBfdyaMsTg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal