To the editor:
Ibrutinib is an irreversible inhibitor of Bruton’s tyrosine kinase (BTK) with promising activity in CD20+ B-cell malignancies including recent US Food and Drug Administration approval in mantle cell lymphoma.1 Given the homology between BTK and interleukin-2 inducible tyrosine kinase (ITK), we recently confirmed that ibrutinib irreversibly binds ITK.2 ITK expression in Fc rceptor (FcR)-stimulated natural killer (NK) cells leads to increased calcium mobilization, granule release, and cytotoxicity.3 As rituximab is a backbone of lymphoma therapy, with mechanisms of action including antibody-dependent cell-mediated cytotoxicity (ADCC), as well as direct induction of apoptosis and complement-dependent cytotoxicity4 and FcR stimulation is requisite for ADCC, we investigated if ibrutinib influenced rituximab’s anti-lymphoma activity in vitro by assessing NK cell interferon-γ secretion, degranulation by CD107a mobilization, and cytotoxicity by chromium release using CD20+ cell lines and autologous patient samples with chronic lymphocytic leukemia (CLL), as well as in xenotransplant lymphoma athymic, ν/ν mouse models, as previously described.5 Trastuzumab and human epidermal growth factor receptor 2+ (HER2+) breast cancer cell lines provided an ADCC control, and CGI-1746, lacking ITK inhibition, represented a BTK selective control.6
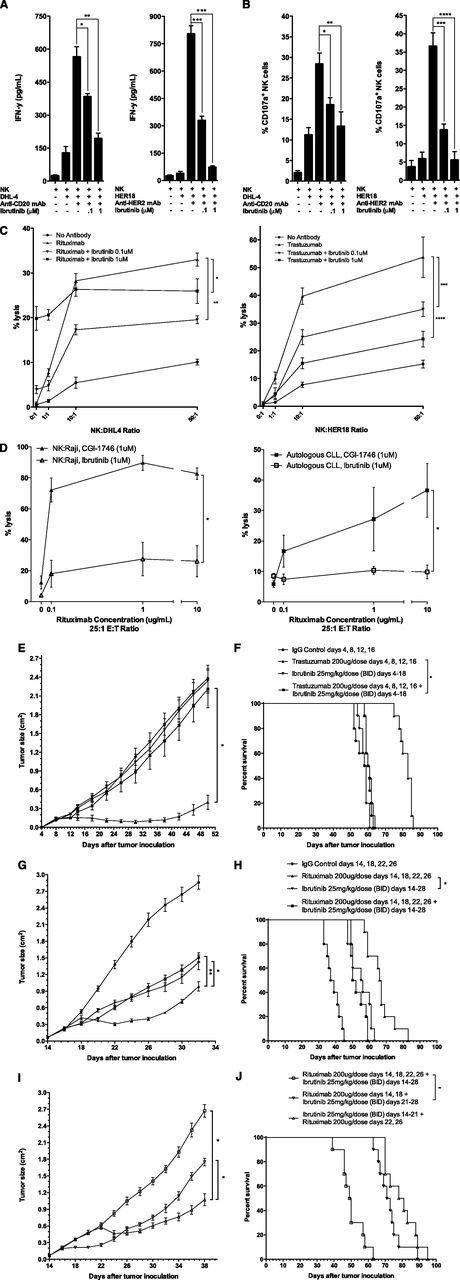
We found that FcR-stimulated NK cells following exposure to rituximab-coated lymphoma cells express high and moderate levels of ITK and BTK, respectively. Ibrutinib inhibited both rituximab- and trastuzumab-induced NK cell cytokine secretion in a dose-dependent manner at 0.1 and 1 μM of ibrutinib in vitro (Figure 1A; *P = .009, **P = .001, ***P < .001). Similarly, ibrutinib prevented FcR-stimulated NK cell degranulation by ∼60% and ∼90% at 0.1 and 1 μM, respectively (Figure 1B; *P = .013, **P = .017, ***P = .002, ****P = .001). Despite direct in vitro cytotoxicity due to ibrutinib independent of NK cells, NK cell–mediated cytotoxicity of both rituximab-coated, chromium-labeled lymphoma cells and trastuzumab-coated, chromium-labeled breast cancer cells was inhibited in an ibrutinib dose-dependent manner (Figure 1C; *P < .001, **P = .045, ***P = .036, ****P = .010). We hypothesize that a dose effect is seen in Figure 1C with trastuzumab and not with ibrutinib as a result of increasing apoptosis, which is a direct dose-dependent effect of ibrutinib monotherapy. Therefore, in vitro, as higher doses of ibrutinib are combined with rituximab, the direct effect of BTK inhibition outweighs the inhibition of NK cell’s ability to perform ADCC. In contrast, in vitro, CGI-1746 had no antagonistic effect on ADCC against rituximab-coated lymphoma cell lines or autologous CLL cells (Figure 1D; *P < .001). Abrogation of trastuzumab-dependent NK cell–mediated cytotoxicity was confirmed in vivo with concurrent ibrutinib daily dosing for 2 weeks during trastuzumab treatment (4 doses), as measured by tumor growth and survival (Figure 1E, *P < .001; Figure 1F, *P = .18). Concurrent ibrutinib daily dosing for 2 weeks during 4 doses of rituximab therapy similarly antagonized rituximab’s efficacy, with anti-lymphoma activity of the combination equivalent to ibrutinib monotherapy (Figure 1G, *P = .049, **P = .032; Figure 1H, *P = .29). Sequential ibrutinib for 1 week followed by 2 doses of rituximab or sequential rituximab (2 doses) followed by ibrutinib for 1 week resulted in restored anti-lymphoma activity superior to concurrent combination therapy of ibrutinib for 2 weeks and 4 doses of rituximab (Figure 1I, *P < .001; Figure 1J, *P < .001).
Ibrutinib antagonizes antibody-dependent NK cell–mediated cytotoxicity. To evaluate NK cell function, purified NK cells were isolated from healthy peripheral blood mononuclear cells and cultured with 0.1 or 1 μM of ibrutinib for 4 hours together with rituximab-coated (10 µg/mL) lymphoma cells, DHL4, or trastuzumab-coated (10 µg/mL) HER2+ breast cancer cells, HER18, and (A) supernatant was harvested and analyzed by enzyme-linked immunosorbent assay for interferon-γ, and (B) NK cells isolated and analyzed for degranulation by flow cytometry for CD107a mobilization. (C-D) NK cell cytotoxicity as percent lysis of DHL4 or HER18 tumor cells was analyzed in chromium release assays with purified NK cells incubated with (C) chromium-labeled DHL4 or HER18 cells for 4 hours at variable effector:target ratios, rituximab (10 µg/mL), and ibrutinib (0.1 or 1 μM) or (D) chromium-labeled Raji or autologous CLL cells for 4 hours at variable rituximab concentrations at a constant effector:target ratio of 25:1 and ibrutinib (1 μM) or CGI-1746 (1 μM). All in vitro experiments were performed in triplicate. To evaluate NK cell function, in vivo athymic ν/ν mouse models (10 mice per group) were xenotransplated with HER18 or DHL4 tumor cells (1 × 106) subcutaneously along the flank on day 0 and monitored for (E,G,I) tumor growth and (F,H,J) survival with experiments performed in duplicate. (E-F) In vivo therapy of the HER18 tumor model included intraperitoneal (ip) immunoglobulin G (IgG) control on days 4, 8, 12, and 16; ip trastuzumab (200 μg) on days 4, 8, 12, and 16; ibrutinib (25 mg/kg/dose) twice daily on days 4 to 18 by oral gavage (og); or the combination. (G-H) In vivo concurrent therapy of the DHL4 lymphoma model included ip IgG control on days 14, 18, 22, and 26; ip rituximab (200 μg) on days 14, 18, 22, and 26, ibrutinib og (25 mg/kg/dose) twice daily on days 14 to 28; or the combination. (I-J) In vivo sequential versus concurrent therapy of the DHL4 lymphoma model included sequential ibrutinib og (25 mg/kg/dose) twice daily on days 14 to 21 and ip rituximab (200 μg) on days 22 and 26; or sequential ip rituximab (200 μg) on days 14 and 18 and ibrutinib og (25 mg/kg/dose) twice daily on days 21 to 28; or concurrent ip rituximab (200 μg) on days 14, 18, 22, and 26 and ibrutinib og (25 mg/kg/dose) twice daily on days 14 to 28. BID, twice daily.
Ibrutinib antagonizes antibody-dependent NK cell–mediated cytotoxicity. To evaluate NK cell function, purified NK cells were isolated from healthy peripheral blood mononuclear cells and cultured with 0.1 or 1 μM of ibrutinib for 4 hours together with rituximab-coated (10 µg/mL) lymphoma cells, DHL4, or trastuzumab-coated (10 µg/mL) HER2+ breast cancer cells, HER18, and (A) supernatant was harvested and analyzed by enzyme-linked immunosorbent assay for interferon-γ, and (B) NK cells isolated and analyzed for degranulation by flow cytometry for CD107a mobilization. (C-D) NK cell cytotoxicity as percent lysis of DHL4 or HER18 tumor cells was analyzed in chromium release assays with purified NK cells incubated with (C) chromium-labeled DHL4 or HER18 cells for 4 hours at variable effector:target ratios, rituximab (10 µg/mL), and ibrutinib (0.1 or 1 μM) or (D) chromium-labeled Raji or autologous CLL cells for 4 hours at variable rituximab concentrations at a constant effector:target ratio of 25:1 and ibrutinib (1 μM) or CGI-1746 (1 μM). All in vitro experiments were performed in triplicate. To evaluate NK cell function, in vivo athymic ν/ν mouse models (10 mice per group) were xenotransplated with HER18 or DHL4 tumor cells (1 × 106) subcutaneously along the flank on day 0 and monitored for (E,G,I) tumor growth and (F,H,J) survival with experiments performed in duplicate. (E-F) In vivo therapy of the HER18 tumor model included intraperitoneal (ip) immunoglobulin G (IgG) control on days 4, 8, 12, and 16; ip trastuzumab (200 μg) on days 4, 8, 12, and 16; ibrutinib (25 mg/kg/dose) twice daily on days 4 to 18 by oral gavage (og); or the combination. (G-H) In vivo concurrent therapy of the DHL4 lymphoma model included ip IgG control on days 14, 18, 22, and 26; ip rituximab (200 μg) on days 14, 18, 22, and 26, ibrutinib og (25 mg/kg/dose) twice daily on days 14 to 28; or the combination. (I-J) In vivo sequential versus concurrent therapy of the DHL4 lymphoma model included sequential ibrutinib og (25 mg/kg/dose) twice daily on days 14 to 21 and ip rituximab (200 μg) on days 22 and 26; or sequential ip rituximab (200 μg) on days 14 and 18 and ibrutinib og (25 mg/kg/dose) twice daily on days 21 to 28; or concurrent ip rituximab (200 μg) on days 14, 18, 22, and 26 and ibrutinib og (25 mg/kg/dose) twice daily on days 14 to 28. BID, twice daily.
The combination of a promising novel agent and current standard of care for the treatment of B-cell lymphomas is currently being explored in multiple phase 2 and 3 trials. Surprisingly, our preclinical investigation of the combination of ibrutinib and rituximab results in antagonistic effects. We demonstrate that the abrogation of both rituximab’s and trastuzumab’s antitumor efficacy is a result of ibrutinib’s inhibition of FcR-stimulated NK cell function, specifically ADCC. Selective BTK inhibitors or alternative ibrutinib dosing schedules, sequential vs concurrent, may preserve the anti-lymphoma efficacy of both agents.
R.L., A.J.J., and J.C.B. contributed equally to this work.
Authorship
Acknowledgments: This work was supported by Specialized Center of Research from the Leukemia and Lymphoma Society, National Cancer Institute grants K12 CA133250, P50 CA140158, P01 CA95426, and P01 CA101956, and The D. Warren Brown Foundation. H.E.K. is supported by the American Society of Hematology, Leukemia and Lymphoma Society, and Damon Runyon Foundation. A.J.J. is a Paul Calabresi Scholar.
Contribution: S.R., S.E.M.H., I.S.-B., and H.E.K. planned the research, performed experiments, analyzed data, drafted the first and subsequent drafts of the letter, and approved the final version of the letter; J.P.B. and C.C. were involved in planning components of the research, performing experiments, reviewed drafts, and approved the final version of the letter; X.Z. was involved in planning components of the research, did all the statistical analysis, reviewed drafts, and approved the final version of the letter; J.J.B. provided input and suggestions to the presentation of the data and a critical reagent (ibrutinib) essential for completion of the work, and reviewed and approved the final version of the letter; N.M. and R.L. provided input into experimental design, reviewed drafts of the manuscript, and approved the final submitted version; and A.J.J. and J.C.B. planned every aspect of the proposal, supervised the research, analyzed data, reviewed drafts, obtained funding for the research work, and approved the final version of the letter.
Conflict-of-interest disclosure: J.J.B. is a former employee of Pharmacyclics, Inc. and has financial interest in ibrutinib development. The remaining authors declare no competing financial interests.
Correspondence: Holbrook Kohrt, Division of Oncology, 269 Campus Dr, CCSR 1145, Stanford University Medical Center, Stanford, CA 94305-5151; e-mail: kohrt@stanford.edu.