To the editor:
β-thalassemia syndromes comprise a global health burden. Hemoglobin E (HbE)–β thalassemia represents 60% of regional populations, with an increasing prevalence in the coastal United States.1,2 Serious complications related to hemolysis and anemia in β-thalassemia intermedia (BTI, or nontransfusion-dependent thalassaemia [NTDT]) are common, with frequent progression to transfusion dependency and alloimmunization.3 There is no effective therapeutic to improve globin balance and reduce anemia for treatment of BTI. Renewed or persistent γ-globin expression is established to compensate for deficient β-globin; affected infants become anemic only after γ-globin suppression and patients with higher fetal hemoglobin (HbF) levels are less anemic than counterparts within genotypes with lower HbF levels.4-8
An oral, nonmutagenic, noncytotoxic short-chain fatty acid derivative, sodium 2,2 dimethylbuyrate (HQK-1001; HemaQuest Pharmaceuticals) induces the γ-globin gene promoter in predictive models, and prolongs erythroid cell survival through enhanced expression of Bcl-xL.9 A dose-ranging trial of HQK-1001 at 10, 20, 30, and 40 mg/kg daily for 8 weeks in 21 BTI subjects with HbE-β0 and β+β0 thalassemia mutations increased HbF by a mean of 6.6% above baseline at 20 mg/kg, the most effective dose.10 This suggested that investigating HQK-1001 treatment of longer periods was warranted.
Accordingly, an open-label trial (NCT01609595), approved by the Ethics Committee of the Department of Medical Science, Ministry of Public Health, Nonthaburi, Thailand, was performed in 10 NTDT patients with HbE-β0 thalassemia, (7 female), ages 20 to 39 years. Subjects received HQK-1001 20 mg/kg once daily for 24 weeks, with 4 weeks’ follow-up observation. Baseline complete blood counts (CBCs) and HbF were obtained twice before dosing and averaged; total Hb ranged from 6 to 8.7 g/dL. CBCs, HbF levels, and safety assessments (including examinations and chemistry panels) were performed monthly, urinalyses bimonthly, and electrocardiograms every 3 months. End points included change from baseline in HbF and total hemoglobin (Hb) as well as safety.
HQK-1001 was well tolerated. Adverse events consisted of mild nausea in 2 subjects, transient elevations in bilirubin, aspartate aminotransferase, and alanine aminotransferase, and 1 case each of tonsillitis and poststreptococcal nephritis.
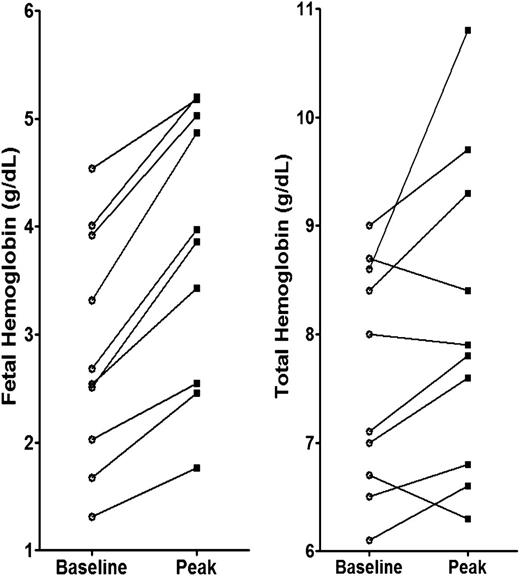
HbF increased in all subjects from a mean of 36.4% to 47.3%; the mean increase in percentage of HbF was 10.9%, (range, 5%-21%; P < .001). Mean absolute HbF increased by 0.96 g/dL, from 2.9 to 3.9 g/dL (P < .002). Total Hb increased in 7 of 10 subjects; the mean change was 0.93 g/dL (range, 0.5-2.2 g/dL; P < .05), as shown in Figure 1. Rises in total Hb began often at week 20, suggesting higher responses might be achieved with longer treatment, particularly in more severely anemic patients, with baseline Hb < 8.0 g/dL. Two subjects who did not achieve a rise in total Hb had infections associated with declines in HbF.
Baseline and peak HbF and total Hb in HbE-β0 thalassemia subjects treated with HQK-1001.
Baseline and peak HbF and total Hb in HbE-β0 thalassemia subjects treated with HQK-1001.
These findings confirm that HQK-1001 increases HbF and total Hb levels in some β-thalassemia syndromes. Longer and alternate regimens, such as intermittent pulsed dosing,5 warrant evaluation to determine its hematologic potential. These findings showing higher responses with longer treatment than in prior trials suggest HQK-1001 merits further investigation in well-characterized β-thalassemia subjects, to determine whether select subsets, and younger subjects with greater hematologic reserve, will respond optimally.
Authorship
Acknowledgments: This work was supported by a Research Chair Grant from the National Science and Technology Institute and Mahidol University and by National Institutes of Health grants (National Institute of Diabetes and Digestive and Kidney Diseases) DK-52962 and (Heart, Lung and Blood Institute) HL-108516.
Contribution: S.P.P. and R.G.G. designed the study and wrote the letter; S.F., M.S.B., and D.H.K.C. analyzed laboratory data; and S.F., N.C., and P.P. treated patients and collected data.
Conflict-of-interest disclosure: R.G.G. is an employee of HemaQuest Pharmaceuticals; S.P.P. is an inventor on patents licensed to, and has equity interest in, HemaQuest Pharmaceuticals; and S.F. has received research support from HemaQuest Phamaceuticals. The remaining authors declare no competing financial interests.
Correspondence: Susan P. Perrine, Boston University School of Medicine, 72 E Concord St, L-908, Boston, MA 02118; e-mail: sperrine@bu.edu.