Key Points
Extended donor Treg survival is required for protection from GVHD; donor Treg longevity depends on Treg CCR8 expression.
Donor CD11c+ APCs promote Treg longevity in vivo; host CD11c+ APCs do not appear to contribute to donor Treg reconstitution.
Abstract
The infusion of donor regulatory T cells (Tregs) has been used to prevent acute graft-versus-host disease (GVHD) in mice and has shown promise in phase 1 clinical trials. Previous work suggested that early Treg migration into lymphoid tissue was important for GVHD prevention. However, it is unclear how and where Tregs function longitudinally to affect GVHD. To better understand their mechanism of action, we studied 2 Treg-associated chemokine receptors in murine stem cell transplant models. CC chemokine receptor (CCR) 4 was dispensable for donor Treg function in the transplant setting. Donor Tregs lacking CCR8 (CCR8−/−), however, were severely impaired in their ability to prevent lethal GVHD because of increased cell death. By itself, CCR8 stimulation was unable to rescue Tregs from apoptosis. Instead, CCR8 potentiated Treg survival by promoting critical interactions with dendritic cells. In vivo, donor bone marrow–derived CD11c+ antigen-presenting cells (APCs) were important for promoting donor Treg maintenance after transplant. In contrast, host CD11c+ APCs appeared to be dispensable for early activation and expansion of donor Tregs. Collectively, our data indicate that a sustained donor Treg presence is critical for their beneficial properties, and that their survival depends on CCR8 and donor but not host CD11c+ APCs.
Introduction
Graft-versus-host disease (GVHD) remains the greatest barrier to successful hematopoietic stem cell transplant (HSCT) outcomes. Over the past several years, substantial animal model research has been devoted to the use of donor, thymically derived, “natural” regulatory T-cell (Treg) infusions for the prevention of GVHD.1-3 In addition, Tregs have recently been administered with both haploidentical and cord blood stem cell products in phase 1 clinical trials.4,5
Despite the growing use of therapeutic Treg infusions in the HSCT setting, it is still unclear how donor Tregs prevent GVHD and which proteins are necessary for their immunosuppressive properties. Although there is indirect evidence that Tregs initially prevent GVHD by blocking early conventional T-cell (Tcon) activation and expansion within recipient lymphoid tissue,6,7 significant uncertainty exists as to how they function later. Understanding how Tregs function over time is critically important to optimizing their clinical use.
CC chemokine receptor (CCR) 4 and CCR8 are both expressed by Tregs and have been shown to mediate the chemotaxis of human Tregs toward dendritic cells (DCs) where they presumably suppress developing immune responses.8 In addition, both receptors have been implicated in the trafficking of Tregs9,10 and T helper 2–polarized Tcons11,12 into the skin, an important GVHD target organ. As a result, we set out to study the contributions of CCR4 and CCR8 to the respective abilities of donor Tcons and Tregs to induce and prevent GVHD.
Using CCR4 and CCR8 knockout mice, we found that neither chemokine receptor alone plays a critical role in GVHD induction by Tcons. Similarly, we found CCR4 to be dispensable for donor Treg function after transplant. CCR8 knockout (CCR8−/−) Tregs, however, were severely impaired in their ability to prevent GVHD because of increased cell death after the first transplant week. Collectively, our data suggest that targeting the CCR8 axis may represent a possible therapeutic approach for either enhancing or reducing Treg numbers in vivo.
Methods
Mice
C57BL/6 (“B6”), B6xDBA/2 F1(“B6D2”), BALB/cJ, B6.PL-Thy1a/CyJ, C.FVB-Tg(Itgax-DTR/EGFP)57Lan/J, and B6.FVB-Tg(Itgax-DTR/EGFP)57Lan/J mice were purchased from The Jackson Laboratory. Enhanced green fluorescent protein (eGFP)–expressing B6 mice were generated as described.13 CCR8−/− mice backcrossed 8 generations onto a B6 background were obtained from the laboratory of Donald Cook.14 All experiments were performed in accordance with protocols approved by the Institutional Animal Care and Use Committee at the University of North Carolina.
Transplantation systems
qRT-PCR
CD4+CD25+ B6 Tregs and CD4+CD25– B6 Tcons were isolated using column purification. Fifty percent of these cells were immediately frozen. The other 50% of the Tregs and Tcons were activated in vitro with plate-bound anti-CD3 and anti-CD28 antibody in the presence of supplemental interleukin (IL) 2 at 100 IU/mL. After 72 hours, these cells were frozen. Later, all cells were thawed, and their total RNA isolated for quantitative reverse-transcription polymerase chain reaction (qRT-PCR) as previously described.15
In vitro suppression assay
Intrinsic Treg immunosuppressive function was assessed using an in vitro suppression assay as previously described.2
Stereomicroscopy
Recipient mice were imaged with a Zeiss StereoLumar V12 microscope with eGFP band-pass filter at room temperature. eGFP intensities were determined with AxioVision (Carl Zeiss) software. For detailed exposure times and magnifications, refer to the supplemental Methods (see the Blood Web site).
Organ eGFP quantification
Recipient organs were homogenized, and absolute eGFP levels measured using an enzyme-linked immunosorbent assay (ELISA) kit (Cell Biolabs).16 We verified that relative eGFP levels within host tissue after transplant correlate with actual Treg numbers in vivo by performing a series of transplants using increasing doses of donor eGFP+ Tregs. As seen in supplemental Figure 1, eGFP levels within host lymphoid tissue as determined by ELISA correlated better with the initial Treg dosing cohort (low, medium, and high) than traditional cell counting by flow cytometry.
Generation of murine BM-derived DCs and macrophages
B6 CD11c+CD14– DCs were generated by culturing B6 BM cells in the presence of supplemental granulocyte macrophage–colony-stimulating factor, IL-4, and tumor necrosis factor as described previously.17 B6 macrophages were generated by culturing B6 BM cells for 7 days in conditioned media obtained from L929 cells (as a macrophage–colony-stimulating factor source) as previously described.18
Statistical methods
Survival curves were constructed using the method of Kaplan and Meier. Overall survival was compared using Fisher’s exact test. For ELISAs, GVHD scoring, and other experiments where distributions were typically not Gaussian, means were compared using the nonparametric Mann-Whitney test. All other continuous variables were compared using the 2-tailed Student t test. Where indicated, Treg ratios following coculture were compared using a 1-sample t test. For this work, the null hypothesis was as follows: the fold-difference results were sampled from a distribution with mean fold difference of 1.0. P values were adjusted for multiple testing according to the methods of Benjamini and Hochberg (false discovery rate control)19 and Bonferroni.20 P values < .05 were considered significant. Error bars represent standard error of the mean.
Results
CCR8 is critical for donor Treg-mediated GVHD protection, but by themselves neither CCR4 nor CCR8 are necessary for lethal GVHD induction
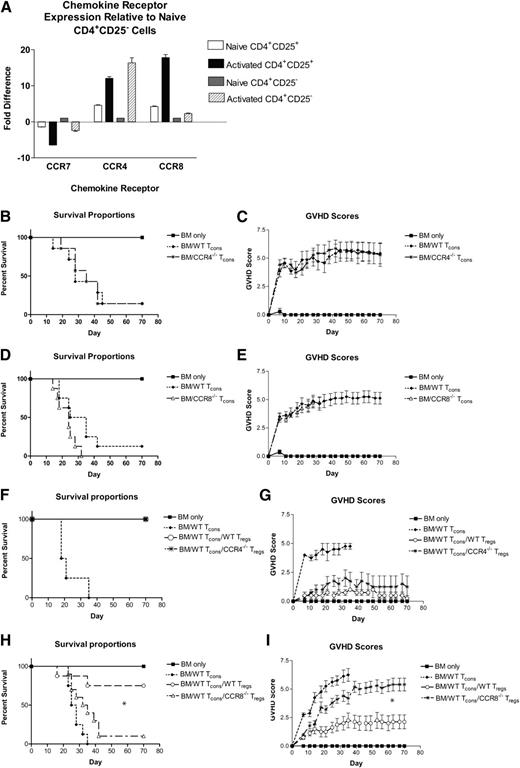
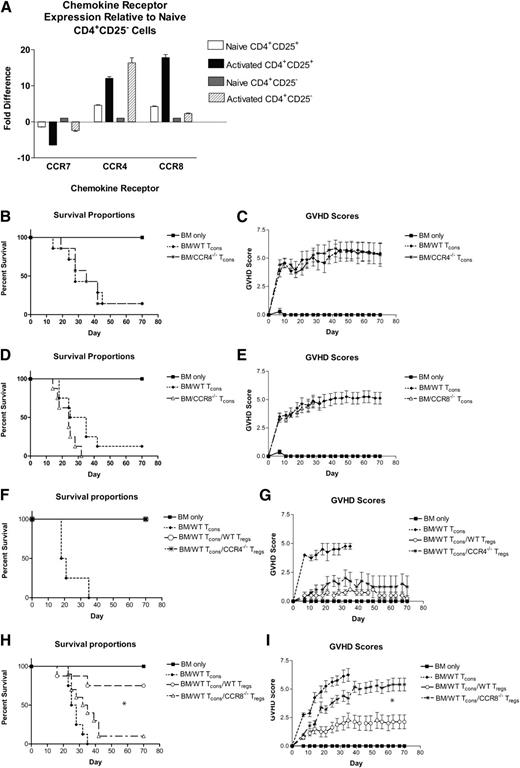
Using qRT-PCR, we examined CCR4 and CCR8 expression by naïve and activated Tcons and Tregs. Compared with naïve CD4+CD25– T cells, both CCR4 and CCR8 were produced at higher levels in unstimulated CD4+CD25+ Tregs (Figure 1A). Upon activation under nonpolarizing conditions, both populations of CD4+ T cells downregulated CCR7 and upregulated CCR4 and CCR8 to varying degrees. We found CCR4 expression to be strongest in activated Tcons, with CCR8 being upregulated preferentially by Tregs.
Critical role for CCR8 expression by Tregs in the prevention of GVHD. (A) Expression of CCR7, CCR4, and CCR8 was evaluated using qRT-PCR in naïve Tregs, activated Tregs, naïve CD4+CD25– Tcons, and activated CD4+ Tcons. Fold differences in expression relative to naïve CD4+CD25– Tcons are depicted. (B-C) B6D2 recipients were lethally irradiated to 950 rads on day –1 and then administered 3 × 106 TCD BM cells from WT B6 donors +/− 4 × 106 CD25-depleted whole (CD4+ and CD8+) splenic Tcons from either WT B6 or CCR4−/− B6 donors on day 0 by tail vein injection. Recipient animals were followed for survival (B) and scored for GVHD twice weekly (C) using a validated clinical scoring system.21 Animals were assigned a score from 0 to 2 for each of 5 GVHD parameters: weight loss, activity, fur ruffling, kyphosis, and skin lesions. Scores ranged from 0 (minimum) to 10 (maximum). Error bars depict standard error of the mean. Data are combined from 2 separate transplant experiments; n = 7 animals per group. (D-E). B6D2 recipients were lethally irradiated to 950 rads on day –1 and then administered 3 × 106 TCD BM cells from WT B6 donors +/− 4 × 106 CD25-depleted splenic Tcons from either WT B6 or CCR8−/− B6 donors on day 0. Data are combined from 2 separate transplant experiments; n = 8 animals per group. (F-G). B6D2 recipients were lethally irradiated on day –1 and then administered 3 × 106 TCD BM cells from WT B6 donors +/− 1 × 106 column-purified CD4+CD25+ Tregs from WT B6 or CCR4−/− B6 mice on day 0. Then 4 × 106 CD25-depleted Tcons from WT B6 donors were administered on day +2; n = 4 animals per group. (H-I) B6D2 recipients were lethally irradiated on day –1 and then administered 3 × 106 TCD BM cells from WT B6 donors +/− 1 × 106 column-purified CD4+CD25+ Tregs from WT B6 or CCR8−/− B6 mice on day 0. Then 4 × 106 CD25-depleted Tcons from WT B6 donors were administered on day +2. Data are combined from 2 separate transplant experiments; n = 4 BM, 8 BM/Tcon, 8 BM/Tcon/WT Treg, 10 BM/Tcon/CCR8−/− Treg. (H) *P = .013 for overall survival comparison between BM/Tcon/WT Treg and BM/Tcon/CCR8−/− Treg groups by Fisher’s exact test. (I) *P = .003 for GVHD score comparison between BM/Tcon/WT Treg and BM/Tcon/CCR8−/− Treg groups on transplant day +70 by the Mann-Whitney Test.
Critical role for CCR8 expression by Tregs in the prevention of GVHD. (A) Expression of CCR7, CCR4, and CCR8 was evaluated using qRT-PCR in naïve Tregs, activated Tregs, naïve CD4+CD25– Tcons, and activated CD4+ Tcons. Fold differences in expression relative to naïve CD4+CD25– Tcons are depicted. (B-C) B6D2 recipients were lethally irradiated to 950 rads on day –1 and then administered 3 × 106 TCD BM cells from WT B6 donors +/− 4 × 106 CD25-depleted whole (CD4+ and CD8+) splenic Tcons from either WT B6 or CCR4−/− B6 donors on day 0 by tail vein injection. Recipient animals were followed for survival (B) and scored for GVHD twice weekly (C) using a validated clinical scoring system.21 Animals were assigned a score from 0 to 2 for each of 5 GVHD parameters: weight loss, activity, fur ruffling, kyphosis, and skin lesions. Scores ranged from 0 (minimum) to 10 (maximum). Error bars depict standard error of the mean. Data are combined from 2 separate transplant experiments; n = 7 animals per group. (D-E). B6D2 recipients were lethally irradiated to 950 rads on day –1 and then administered 3 × 106 TCD BM cells from WT B6 donors +/− 4 × 106 CD25-depleted splenic Tcons from either WT B6 or CCR8−/− B6 donors on day 0. Data are combined from 2 separate transplant experiments; n = 8 animals per group. (F-G). B6D2 recipients were lethally irradiated on day –1 and then administered 3 × 106 TCD BM cells from WT B6 donors +/− 1 × 106 column-purified CD4+CD25+ Tregs from WT B6 or CCR4−/− B6 mice on day 0. Then 4 × 106 CD25-depleted Tcons from WT B6 donors were administered on day +2; n = 4 animals per group. (H-I) B6D2 recipients were lethally irradiated on day –1 and then administered 3 × 106 TCD BM cells from WT B6 donors +/− 1 × 106 column-purified CD4+CD25+ Tregs from WT B6 or CCR8−/− B6 mice on day 0. Then 4 × 106 CD25-depleted Tcons from WT B6 donors were administered on day +2. Data are combined from 2 separate transplant experiments; n = 4 BM, 8 BM/Tcon, 8 BM/Tcon/WT Treg, 10 BM/Tcon/CCR8−/− Treg. (H) *P = .013 for overall survival comparison between BM/Tcon/WT Treg and BM/Tcon/CCR8−/− Treg groups by Fisher’s exact test. (I) *P = .003 for GVHD score comparison between BM/Tcon/WT Treg and BM/Tcon/CCR8−/− Treg groups on transplant day +70 by the Mann-Whitney Test.
We next examined how CCR4 and CCR8 would affect the ability of donor Tcons to induce GVHD. For these experiments, we used a well-described C57BL/6 (H-2b; “B6”) into B6xDBA/2 F1 (H-2bxd; “B6D2”) haplotype matched murine transplant model and donor B6 mice knocked out at either the CCR4 (Figure 1B-C) or CCR8 locus (Figure 1D-E). Both CCR4−/− and CCR8−/− Tcons induced severe GVHD in wild-type (WT) B6D2 recipients that was virtually indistinguishable from that generated by WT Tcons.21
Because both CCR4 and CCR8 are expressed by naïve and in particular activated Tregs, we next examined the impact that these receptors would have on the ability of donor Tregs to prevent lethal GVHD. Somewhat unexpectedly, we found that CCR4−/− Tregs were efficient at attenuating GVHD, with 100% of CCR4−/− Treg recipients surviving until the end of the study (Figure 1F-G). CCR8−/− Treg recipients, however, developed severe GVHD in a very reproducible pattern (Figure 1H-I). For the first 7 to 14 days posttransplant, recipients of CCR8−/− Tregs exhibited low GVHD scores similar to those mice given WT Tregs. Thereafter, however, the GVHD curves between the CCR8−/− and WT Treg treatment groups diverged with recipients of CCR8−/− Tregs developing acute GVHD that ultimately approximated that of control mice receiving Tcons alone (Figure 1I). In summary, although Tregs lacking CCR8 were initially effective in preventing GVHD, those animals given CCR8−/− Tregs developed delayed, severe disease.
CCR8−/− Tregs undergo normal activation and proliferation in vivo and demonstrate preserved immunosuppressive properties in vitro
To elucidate a mechanism for the impaired capacity of CCR8−/− Tregs to prevent GVHD lethality, we examined their activation and expansion early after transfer. Regardless of CCR8 expression, donor Tregs demonstrated a primarily activated phenotype by transplant day +7, with reduced levels of l-selectin and abundant CD44 (Figure 2A-B).
Absence of CCR8 does not impair Treg activation or suppressive function. (A-B) B6D2 mice were lethally irradiated on day −1 and then transplanted with 3 × 106 WT B6 TCD BM cells with either 1 × 106 WT eGFP+ B6 or CCR8−/− eGFP+ B6 Tregs on day 0. Then 4 × 106 WT eGFP– B6 Tcons were administered on day +2. On transplant day +7, recipient mice were euthanized, and their spleens disrupted. Donor Tregs were then examined for activation markers using a Foxp3+eGFP+ gate. Representative flow cytometry plots following l-selectin (A) and CD44 (B) staining are depicted. In addition, the mean percentages of l-selectinlow and CD44high cells are depicted graphically; n = 9 total recipients per group. The spleens were pooled into groups of 3 to maximize the number of donor events, and mean percentages of the groups compared using the Mann-Whitney Test. (C) 1.5 × 106 freshly isolated CD4+CD25+ WT or CCR8−/− Tregs were labeled with CFSE and transplanted with 3 × 106 unlabeled WT B6 TCD BM cells into irradiated B6D2 recipients. On transplant day +5, recipient mice were euthanized, their spleens disrupted, and donor Tregs examined for CFSE positivity by flow cytometry using a Kd–, Foxp3+ gate. (D) CD4+CD25+ Tregs were isolated from WT or CCR8−/− B6 mice and then cultured for 72 hours at varying ratios with 5 × 104 CD4+CD25– WT B6 responder cells and 5 × 104 irradiated TCD B6D2 stimulator splenocytes. During the last 16 to 20 hours of incubation, 0.037 MBq (1 µCi) of [3H]thymidine was added to each well, and [3H]thymidine incorporation measured by scintillation counting.
Absence of CCR8 does not impair Treg activation or suppressive function. (A-B) B6D2 mice were lethally irradiated on day −1 and then transplanted with 3 × 106 WT B6 TCD BM cells with either 1 × 106 WT eGFP+ B6 or CCR8−/− eGFP+ B6 Tregs on day 0. Then 4 × 106 WT eGFP– B6 Tcons were administered on day +2. On transplant day +7, recipient mice were euthanized, and their spleens disrupted. Donor Tregs were then examined for activation markers using a Foxp3+eGFP+ gate. Representative flow cytometry plots following l-selectin (A) and CD44 (B) staining are depicted. In addition, the mean percentages of l-selectinlow and CD44high cells are depicted graphically; n = 9 total recipients per group. The spleens were pooled into groups of 3 to maximize the number of donor events, and mean percentages of the groups compared using the Mann-Whitney Test. (C) 1.5 × 106 freshly isolated CD4+CD25+ WT or CCR8−/− Tregs were labeled with CFSE and transplanted with 3 × 106 unlabeled WT B6 TCD BM cells into irradiated B6D2 recipients. On transplant day +5, recipient mice were euthanized, their spleens disrupted, and donor Tregs examined for CFSE positivity by flow cytometry using a Kd–, Foxp3+ gate. (D) CD4+CD25+ Tregs were isolated from WT or CCR8−/− B6 mice and then cultured for 72 hours at varying ratios with 5 × 104 CD4+CD25– WT B6 responder cells and 5 × 104 irradiated TCD B6D2 stimulator splenocytes. During the last 16 to 20 hours of incubation, 0.037 MBq (1 µCi) of [3H]thymidine was added to each well, and [3H]thymidine incorporation measured by scintillation counting.
We next evaluated the ability of CCR8−/− Tregs to proliferate in vivo using carboxyfluorescein diacetate succinimidyl ester (CFSE) labeling. For this experiment, freshly isolated WT or CCR8−/− Tregs were labeled with CFSE and then transplanted into irradiated B6D2 recipients. As seen in Figure 2C, both WT and CCR8−/− donor Tregs demonstrated a near total loss of CFSE, consistent with the very aggressive homeostatic Treg expansion that occurs in the lymphopenic environment that develops after lethal conditioning. CFSE labeling controls using permeabilized cells demonstrated no loss of CFSE signal intensity as a result of the permeabilization step required for intracellular Foxp3 staining (supplemental Figure 2).
Next, we examined the ability of WT and CCR8−/− Tregs to suppress CD4+ T-cell proliferation in a 1-way mixed lymphocyte reaction (Figure 2D). Using irradiated TCD B6D2 splenocytes as stimulator cells, we were unable to detect a difference in the in vitro suppressive ability of CCR8−/− vs WT Tregs at the dilutions examined. Taken together, these data indicated that without CCR8, natural Tregs remain capable of normal activation in vivo and possess no intrinsic defects in their ability to suppress CD4+ T-cell proliferation.
Tregs lacking CCR8 accumulate efficiently within recipient tissues but demonstrate abnormal longevity
Because chemokine receptors are known to contribute to lymphocyte trafficking in vivo, we hypothesized that CCR8−/− Tregs might demonstrate abnormal homing after transplant. To address this question, irradiated B6D2 recipients were administered WT or CCR8−/− eGFP+ Tregs. Treg trafficking into recipient lymph nodes and Peyer’s patches was then assessed at multiple time points after transplant using fluorescence stereomicroscopy. Additionally, we used an anti-eGFP ELISA to compare relative donor Treg numbers within GVHD target organs between WT and CCR8−/− recipients.16
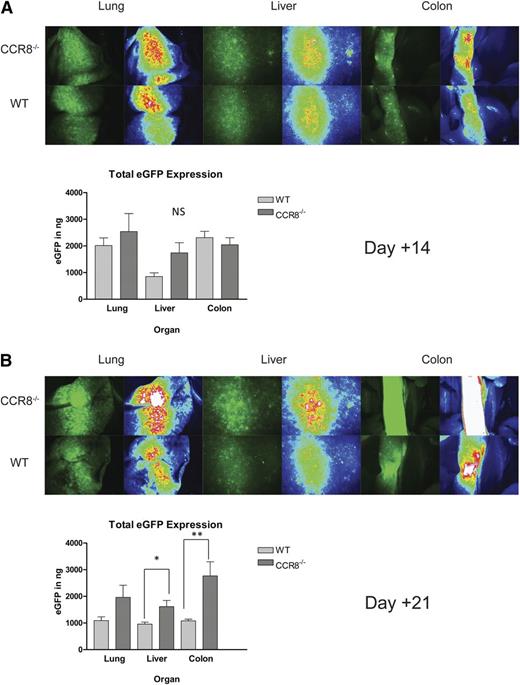
As seen in Figure 3A, by posttransplant day +7, WT and CCR8−/− Tregs accumulated to a similar degree within the inguinal lymph nodes, mesenteric lymph nodes, and Peyer’s patches. In addition, similar eGFP levels were detected by ELISA within the spleen and peripheral GVHD target organs including the liver, lung, and colon. Notably, the strength of the eGFP signal at this relatively early time point was unexpectedly intense within the colon in both treatment groups, suggesting a particularly strong Treg tropism for gastrointestinal tissues in the HSCT setting.
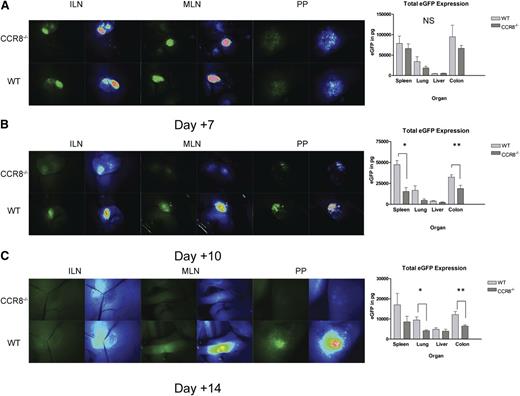
Decreased accumulation of CCR8 −/− Tregs after transplant day +7. (A-C). B6D2 recipients were lethally irradiated on day –1 and then administered 3 × 106 TCD BM cells from WT eGFP– B6 donors plus 1 × 106 column-purified CD4+CD25+ Tregs from WT eGFP+ B6 or CCR8−/− eGFP+ B6 mice on day 0. Then 4 × 106 CD25-depleted eGFP– Tcons from WT B6 donors were administered on day +2. Recipient animals were anesthetized on transplant days +7 (A), +10 (B), or +14 (C), and lymphoid organs imaged by fluorescence stereomicroscopy. Total eGFP levels were then quantified in these sites using an eGFP ELISA kit. (A) n = 4 recipients per group. (B) n = 5 recipients per group. *P = .008 for total eGFP comparison between WT and CCR8−/− Treg recipients by the Mann-Whitney test. **P = .032. (C) n = 8 WT Treg and 5 CCR8−/− Treg recipients. *P = .019; **P = .011.
Decreased accumulation of CCR8 −/− Tregs after transplant day +7. (A-C). B6D2 recipients were lethally irradiated on day –1 and then administered 3 × 106 TCD BM cells from WT eGFP– B6 donors plus 1 × 106 column-purified CD4+CD25+ Tregs from WT eGFP+ B6 or CCR8−/− eGFP+ B6 mice on day 0. Then 4 × 106 CD25-depleted eGFP– Tcons from WT B6 donors were administered on day +2. Recipient animals were anesthetized on transplant days +7 (A), +10 (B), or +14 (C), and lymphoid organs imaged by fluorescence stereomicroscopy. Total eGFP levels were then quantified in these sites using an eGFP ELISA kit. (A) n = 4 recipients per group. (B) n = 5 recipients per group. *P = .008 for total eGFP comparison between WT and CCR8−/− Treg recipients by the Mann-Whitney test. **P = .032. (C) n = 8 WT Treg and 5 CCR8−/− Treg recipients. *P = .019; **P = .011.
When we performed a similar set of experiments on transplant day +10, we began to observe differences between the 2 treatment groups. By microscopy, fewer CCR8−/− Tregs were noted within recipient inguinal lymph nodes, mesenteric lymph nodes, and Peyer’s patches, and by ELISA significantly less eGFP was detected within the spleen and colon (Figure 3B). By day +14, a weaker eGFP signal was again visualized within recipient lymphatic tissue in those mice given CCR8−/− Tregs, and less eGFP was detected within the lung and colon (Figure 3C). Collectively, these data indicated that despite a preserved ability to accumulate within host tissues early after transplant, CCR8−/− Tregs failed to persist in vivo to the same extent as WT cells.
Recipients of CCR8−/− Tregs exhibit a delayed overgrowth of donor Tcons within host tissues
We next assessed how the premature loss of CCR8−/− donor Tregs would affect donor Tcon numbers within recipient tissues. For these experiments, WT or CCR8−/− eGFP– Tregs were transplanted into irradiated B6D2 recipients along with eGFP+ B6 Tcons. As seen in Figure 4A, recipients of WT and CCR8−/− Tregs demonstrated similar Tcon accumulation within peripheral GVHD target organs 2 weeks after transplant. Twenty-one days after transplantation, however, those mice receiving CCR8−/− Tregs developed a delayed increase of Tcons within multiple sites by microscopy, and significantly greater eGFP levels within the liver and the colon by ELISA (Figure 4B). In summary, WT and CCR8−/− Tregs allowed for a similar degree of donor Tcon expansion over the first 1 to 2 weeks after transplant, which corresponded to the nearly identical clinical GVHD scores observed in the 2 groups over the same time period (Figure 1I). Thereafter, the accelerated decline in donor Treg numbers seen in mice given CCR8−/− cells was accompanied by an increase of Tcons in several GVHD target organs.
Increased donor Tcon accumulation within host tissues in recipients of CCR8 −/− Tregs. (A-B) B6D2 recipients were lethally irradiated on day −1 and then administered 3 × 106 TCD BM cells from WT B6 donors plus 1 × 106 column-purified CD4+CD25+ Tregs from WT eGFP– B6 or CCR8−/− eGFP– B6 mice on day 0. Then 4 × 106 CD25-depleted eGFP+ Tcons from WT B6 donors were administered on day +2. Recipient animals were anesthetized on transplant days +14 (A) and day +21 (B), and their organs imaged by fluorescence stereomicroscopy. Total eGFP levels were then quantified in these sites using an eGFP ELISA kit. (A) n = 4 recipients per group. (B) n = 5 recipients per group. *P = .032 for total eGFP comparison between WT and CCR8−/− Treg recipients by the Mann-Whitney test. **P = .016.
Increased donor Tcon accumulation within host tissues in recipients of CCR8 −/− Tregs. (A-B) B6D2 recipients were lethally irradiated on day −1 and then administered 3 × 106 TCD BM cells from WT B6 donors plus 1 × 106 column-purified CD4+CD25+ Tregs from WT eGFP– B6 or CCR8−/− eGFP– B6 mice on day 0. Then 4 × 106 CD25-depleted eGFP+ Tcons from WT B6 donors were administered on day +2. Recipient animals were anesthetized on transplant days +14 (A) and day +21 (B), and their organs imaged by fluorescence stereomicroscopy. Total eGFP levels were then quantified in these sites using an eGFP ELISA kit. (A) n = 4 recipients per group. (B) n = 5 recipients per group. *P = .032 for total eGFP comparison between WT and CCR8−/− Treg recipients by the Mann-Whitney test. **P = .016.
The accelerated in vivo decline of donor Tregs lacking CCR8 is because of an increased susceptibility to cell death rather than a defect in their proliferative capacity
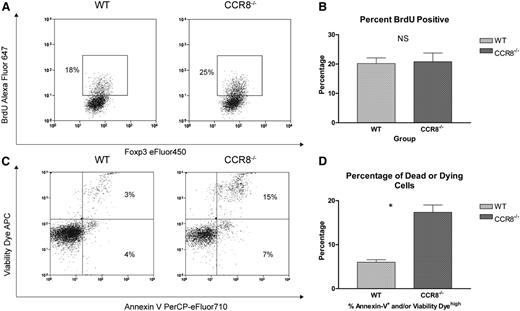
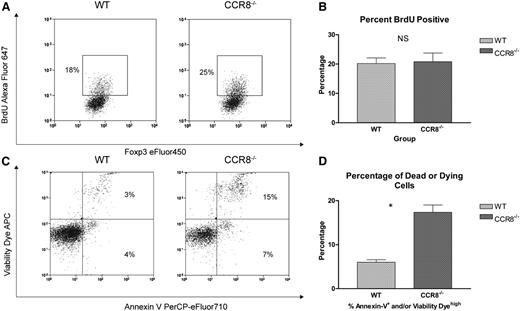
Based on our previous findings, we hypothesized that the accelerated in vivo decline in donor CCR8−/− Tregs was the result of either a deficiency in their capacity to undergo extended cell division after transplant or an increased susceptibility to cell death. To evaluate for any differences in the late proliferative potential of WT vs CCR8−/− donor Tregs after transplant, a 5-bromo-2’-deoxyuridine (BrdU) labeling approach was used. As seen in Figure 5A-B, no differences in BrdU incorporation were noted between recipients of WT and CCR8−/− Tregs on transplant day +14.
CCR8 −/− Tregs undergo enhanced apoptosis. (A-B) B6D2 recipients were lethally irradiated on day −1 and then administered 3 × 106 TCD BM cells from WT B6 donors plus 1 × 106 column-purified CD4+CD25+ Tregs from WT eGFP+ B6 or CCR8−/− eGFP+ B6 mice on day 0. Then 4 × 106 CD25-depleted eGFP– Tcons from WT B6 donors were administered on day +2. On transplant day +14, recipient animals were each administered 250 µL of BrdU (Invitrogen) by intraperitoneal injection. After 3 hours, these mice were euthanized, their spleens disrupted, and the cells stained with an anti-BrdU antibody. Donor Tregs within the recipient spleen were then evaluated for BrdU uptake by flow cytometry using an eGFP+Foxp3+ gate. (A) Representative flow cytometry plots of a WT and CCR8−/− Treg recipient are depicted. (B) The percentages of BrdU+ Tregs are depicted graphically; n = 5 mice per treatment group. (C-D) B6D2 recipients were lethally irradiated on day −1 and then administered 3 × 106 TCD BM cells from WT eGFP– B6 donors plus 1 × 106 column-purified CD4+CD25+ Tregs from WT eGFP+ B6 or CCR8−/− eGFP+ B6 mice on day 0. Then 4 × 106 CD25-depleted eGFP– Tcons from WT B6 donors were administered on day +2. On transplant day +10, recipient mice were euthanized, and their spleens disrupted. Donor Tregs within the recipient spleen were then examined for markers of apoptosis and cell viability by flow cytometry using an eGFP+Foxp3+ gate. A commercially available amine reactive viability dye was used, as standard propidium iodide staining is not compatible with the membrane permeabilization step required for intracellular Foxp3 staining. (C) Representative flow cytometry plots of a WT and CCR8−/− Treg recipient are depicted. (D) The percentages of dead or dying donor Tregs (Annexin Vhigh and/or Viability Dyehigh) are depicted graphically; n = 3 mice per treatment group. *P = .003 by Student t test.
CCR8 −/− Tregs undergo enhanced apoptosis. (A-B) B6D2 recipients were lethally irradiated on day −1 and then administered 3 × 106 TCD BM cells from WT B6 donors plus 1 × 106 column-purified CD4+CD25+ Tregs from WT eGFP+ B6 or CCR8−/− eGFP+ B6 mice on day 0. Then 4 × 106 CD25-depleted eGFP– Tcons from WT B6 donors were administered on day +2. On transplant day +14, recipient animals were each administered 250 µL of BrdU (Invitrogen) by intraperitoneal injection. After 3 hours, these mice were euthanized, their spleens disrupted, and the cells stained with an anti-BrdU antibody. Donor Tregs within the recipient spleen were then evaluated for BrdU uptake by flow cytometry using an eGFP+Foxp3+ gate. (A) Representative flow cytometry plots of a WT and CCR8−/− Treg recipient are depicted. (B) The percentages of BrdU+ Tregs are depicted graphically; n = 5 mice per treatment group. (C-D) B6D2 recipients were lethally irradiated on day −1 and then administered 3 × 106 TCD BM cells from WT eGFP– B6 donors plus 1 × 106 column-purified CD4+CD25+ Tregs from WT eGFP+ B6 or CCR8−/− eGFP+ B6 mice on day 0. Then 4 × 106 CD25-depleted eGFP– Tcons from WT B6 donors were administered on day +2. On transplant day +10, recipient mice were euthanized, and their spleens disrupted. Donor Tregs within the recipient spleen were then examined for markers of apoptosis and cell viability by flow cytometry using an eGFP+Foxp3+ gate. A commercially available amine reactive viability dye was used, as standard propidium iodide staining is not compatible with the membrane permeabilization step required for intracellular Foxp3 staining. (C) Representative flow cytometry plots of a WT and CCR8−/− Treg recipient are depicted. (D) The percentages of dead or dying donor Tregs (Annexin Vhigh and/or Viability Dyehigh) are depicted graphically; n = 3 mice per treatment group. *P = .003 by Student t test.
Next, we looked for any differences in Treg survival that might occur in the absence of CCR8. For this work, irradiated B6D2 mice were transplanted with Tregs from either WT or CCR8−/− donors. On transplant day +10, donor Tregs were then examined for markers of apoptosis and cell viability. As seen in Figure 5C-D, CCR8−/− donor Tregs were found to be more susceptible to apoptosis after adoptive transfer, with 18% of the CCR8−/− cells undergoing cell death compared with only 7% of the WT Tregs at this time point.
CCR8 improves Treg survival in vitro in the presence of activated syngeneic DCs
Given the survival defect displayed by CCR8−/− Tregs, we questioned whether either of CCR8’s known agonists, CC chemokine ligand (CCL) 1 or CCL8,22 could directly affect Treg survival in vitro. Previously, Tregs have been shown to be sensitive to apoptosis when cultured without supplemental IL-2.23,24 Taking advantage of this, we cultured WT B6 Tregs on plates coated overnight with anti-CD3 and anti-CD28 antibody. In the setting of T-cell receptor and CD28 stimulation, more than half of the Tregs survived to 72 hours. Nevertheless, neither the addition of supplemental CCL1 nor CCL8 over a range of physiological concentrations in the absence of IL-2 had any direct effects on Treg viability (supplemental Figure 3).
Previous studies have demonstrated a role for DCs in the survival of Tregs in nontransplanted mice.25 Also, DCs have been shown to produce CCL1, a modest but highly specific chemoattractant for Tregs.8 As a result, we hypothesized that CCR8 signaling might enhance extended Treg survival by facilitating crucial interactions with syngeneic donor DCs. To test this hypothesis, we generated activated CD11c+ Class II MHChigh CD14– DCs from B6 BM cells.17 Using qRT-PCR, we found that B6 DCs produced both CCL1 and CCL8 (supplemental Figure 4A) and verified that CCL1 but not CCL8 was able to induce Treg chemotaxis in a CCR8-dependent fashion (supplemental Figure 4B).
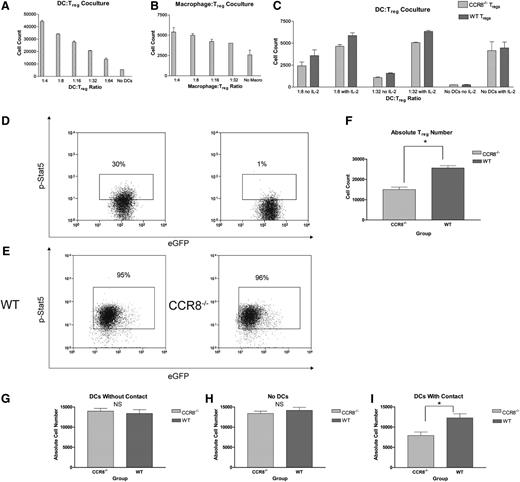
Following up on these data, we determined whether we could prevent B6 Treg apoptosis in vitro with B6 DCs. For these experiments, we cocultured activated B6 DCs with freshly isolated B6 Tregs on uncoated plates in the absence of supplemental IL-2. As seen in Figure 6A, activated DCs were able to rescue syngeneic WT Tregs from cell death in a dose-dependent fashion. In addition, we examined whether this process was specific to DCs by culturing B6 Tregs with syngeneic B6 macrophages. As seen in Figure 6B, macrophages were modestly able to prevent Treg apoptosis but were generally less efficient and less potent than DCs on a per cell basis. As a result, we elected to focus exclusively on Treg:DC interactions for the remainder of our experiments.
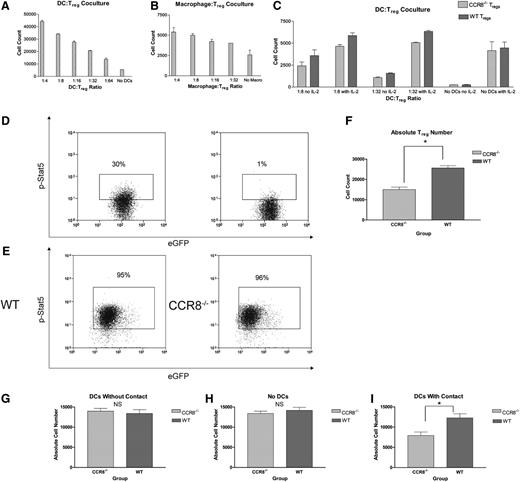
Absence of CCR8 leads to diminished Treg numbers in the presence of syngeneic DCs in vitro. (A) CD4+CD25+ WT B6 Tregs (5 × 105) were cultured for 72 hours on fibronectin-coated dishes in IL-2–deficient media with varying dilutions of syngeneic WT B6 BM-derived DCs. Cells were subsequently stained with an amine-reactive viability dye, and the total number of viable Tregs determined by flow cytometry. All wells were done in duplicate. (B) CD4+CD25+ WT B6 Tregs (5 × 105) were cultured for 72 hours on fibronectin-coated dishes in IL-2–deficient media with varying dilutions of syngeneic WT B6 BM-derived macrophages. Cells were subsequently stained with an amine-reactive viability dye, and the total number of viable Tregs determined by flow cytometry. All wells were done in duplicate. (C) CD4+CD25+ WT Thy1.1+ eGFP– B6 Tregs (5 × 105) were incubated for 72 hours with an equal number of CCR8−/− Thy1.2+ eGFP+ B6 Tregs with or without varying dilutions of syngeneic WT eGFP– B6 DCs in the presence or absence of supplemental IL-2 at 100 IU/mL. Absolute numbers of viable WT (Thy1.1+) and CCR8−/− (eGFP+) Tregs were subsequently measured by flow cytometry using a Foxp3+Viability Dyelow gate, and the ratio of CCR8−/− to WT Tregs then determined for each well. The ratio of CCR8−/− Tregs to WT Tregs did not vary according to the presence or absence of IL-2 for any of the 3 DC:Treg culture conditions (1:8, 1:32, no DCs). As a result, all of the CCR8−/− Treg to WT Treg ratios from a given culture condition (with and without IL-2) were pooled and compared with a reference ratio of 1.0 using a 1-sample t test; n = 4 per culture condition. P = .013 for 1:8 cultures; P = .017 for 1:32 cultures; P = 1.0 for no DC cultures. (D) Freshly isolated CD4+CD25+ WT eGFP+ B6 Tregs (1 × 106) were fixed in a 1% formaldehyde solution and then permeabilized with a commercially available buffer (Becton Dickinson). Cells were subsequently stained with a phycoerythrin-conjugated antibody against the phosphorylated form of Stat5 (left panel). As a negative control, freshly isolated WT eGFP+ B6 Tregs were stained for phospho-Stat5 but without the preceding nuclear membrane permeabilization step (right panel). (E) CD4+CD25+ WT eGFP+ B6 Tregs or CCR8−/− eGFP+ B6 Tregs (5 × 105) were incubated with 5 × 104 WT eGFP– B6 DCs. After 72 hours, the cells were fixed and permeabilized. Cells were subsequently stained for phospho-Stat5. The buffers needed for nuclear phosphorylated transcription factor staining are poorly compatible with cell surface and Foxp3 staining. As a result, Tregs were distinguished from DCs using a forward vs side scatter lymphocyte gate and by nature of their eGFP positivity. Representative flow plots of WT (left) and CCR8−/− Tregs (right) are depicted. (F) CD4+CD25+ WT eGFP+ B6 Tregs or CCR8−/− eGFP+ B6 Tregs (5 × 105) were incubated with 5 × 104 WT eGFP– B6 DCs for 72 hours. Absolute numbers of WT and CCR8−/− Tregs were then determined by flow cytometry using an eGFP+ lymphocyte gate; n = 5 WT Treg, n = 6 CCR8−/− Treg. *P = .001 by Student t test. (G-I) CD4+CD25+ WT eGFP+ B6 Tregs or CCR8−/− eGFP+ B6 Tregs (5 × 105) were seeded in the lower wells of 24-well 3-µM transwell plates and cultured for 72 hours with either 5 × 104 WT eGFP– B6 DCs placed in the upper chambers (G), with no DCs (H), or with 5 × 104 WT eGFP– B6 DCs placed also in the lower chambers (I). Absolute numbers of WT and CCR8−/− Tregs were then determined by flow cytometry using an eGFP+ lymphocyte gate; n = 4 per group. *P = .018 by Student t test.
Absence of CCR8 leads to diminished Treg numbers in the presence of syngeneic DCs in vitro. (A) CD4+CD25+ WT B6 Tregs (5 × 105) were cultured for 72 hours on fibronectin-coated dishes in IL-2–deficient media with varying dilutions of syngeneic WT B6 BM-derived DCs. Cells were subsequently stained with an amine-reactive viability dye, and the total number of viable Tregs determined by flow cytometry. All wells were done in duplicate. (B) CD4+CD25+ WT B6 Tregs (5 × 105) were cultured for 72 hours on fibronectin-coated dishes in IL-2–deficient media with varying dilutions of syngeneic WT B6 BM-derived macrophages. Cells were subsequently stained with an amine-reactive viability dye, and the total number of viable Tregs determined by flow cytometry. All wells were done in duplicate. (C) CD4+CD25+ WT Thy1.1+ eGFP– B6 Tregs (5 × 105) were incubated for 72 hours with an equal number of CCR8−/− Thy1.2+ eGFP+ B6 Tregs with or without varying dilutions of syngeneic WT eGFP– B6 DCs in the presence or absence of supplemental IL-2 at 100 IU/mL. Absolute numbers of viable WT (Thy1.1+) and CCR8−/− (eGFP+) Tregs were subsequently measured by flow cytometry using a Foxp3+Viability Dyelow gate, and the ratio of CCR8−/− to WT Tregs then determined for each well. The ratio of CCR8−/− Tregs to WT Tregs did not vary according to the presence or absence of IL-2 for any of the 3 DC:Treg culture conditions (1:8, 1:32, no DCs). As a result, all of the CCR8−/− Treg to WT Treg ratios from a given culture condition (with and without IL-2) were pooled and compared with a reference ratio of 1.0 using a 1-sample t test; n = 4 per culture condition. P = .013 for 1:8 cultures; P = .017 for 1:32 cultures; P = 1.0 for no DC cultures. (D) Freshly isolated CD4+CD25+ WT eGFP+ B6 Tregs (1 × 106) were fixed in a 1% formaldehyde solution and then permeabilized with a commercially available buffer (Becton Dickinson). Cells were subsequently stained with a phycoerythrin-conjugated antibody against the phosphorylated form of Stat5 (left panel). As a negative control, freshly isolated WT eGFP+ B6 Tregs were stained for phospho-Stat5 but without the preceding nuclear membrane permeabilization step (right panel). (E) CD4+CD25+ WT eGFP+ B6 Tregs or CCR8−/− eGFP+ B6 Tregs (5 × 105) were incubated with 5 × 104 WT eGFP– B6 DCs. After 72 hours, the cells were fixed and permeabilized. Cells were subsequently stained for phospho-Stat5. The buffers needed for nuclear phosphorylated transcription factor staining are poorly compatible with cell surface and Foxp3 staining. As a result, Tregs were distinguished from DCs using a forward vs side scatter lymphocyte gate and by nature of their eGFP positivity. Representative flow plots of WT (left) and CCR8−/− Tregs (right) are depicted. (F) CD4+CD25+ WT eGFP+ B6 Tregs or CCR8−/− eGFP+ B6 Tregs (5 × 105) were incubated with 5 × 104 WT eGFP– B6 DCs for 72 hours. Absolute numbers of WT and CCR8−/− Tregs were then determined by flow cytometry using an eGFP+ lymphocyte gate; n = 5 WT Treg, n = 6 CCR8−/− Treg. *P = .001 by Student t test. (G-I) CD4+CD25+ WT eGFP+ B6 Tregs or CCR8−/− eGFP+ B6 Tregs (5 × 105) were seeded in the lower wells of 24-well 3-µM transwell plates and cultured for 72 hours with either 5 × 104 WT eGFP– B6 DCs placed in the upper chambers (G), with no DCs (H), or with 5 × 104 WT eGFP– B6 DCs placed also in the lower chambers (I). Absolute numbers of WT and CCR8−/− Tregs were then determined by flow cytometry using an eGFP+ lymphocyte gate; n = 4 per group. *P = .018 by Student t test.
To more definitively evaluate whether CCR8 would affect the ability of DCs to promote Treg survival in vitro, we cocultured equal numbers of WT eGFP– Thy1.1+ B6 Tregs and CCR8−/− eGFP+ Thy1.2+ Tregs with variable numbers of eGFP– activated B6 DCs. Under these circumstances, the WT and CCR8−/− Tregs were present in the same culture conditions and therefore forced to compete with one another for access to prosurvival signals provided by the resident syngeneic DCs. After 72 hours, Treg numbers were determined by flow cytometry. As seen in Figure 6C, no difference in the ratio of WT to CCR8−/− Tregs was observed in the absence of DCs. However, when DCs were added to the culture conditions, we consistently found an increase in the number of WT Tregs relative to the number of CCR8−/− Tregs, suggesting that CCR8 facilitates prosurvival interactions between Tregs and resident DCs. Notably, this effect was observed even in the presence of saturating amounts of supplemental IL-2, a cytokine critical for Treg development and growth. Because the WT and CCR8−/− Tregs were admixed under these culture conditions and therefore exposed to the same soluble signaling milieu, our data suggested that WT Tregs are better able to respond to DCs locally in an IL-2–independent fashion.
To further explore the mechanism by which syngeneic DCs are able to promote Treg survival, we examined B6 Treg signal transducer and activator of transcription (STAT) 5 phosphorylation following coincubation with B6 DCs. Stat5 is an important signaling intermediary downstream of both the T-cell receptor26 and the IL-2 receptor,27 and it is known to mediate antiapoptotic affects within Tregs via the induction of Bcl-xL.27 In addition, Stat5 directly activates Foxp3 expression and inhibits T helper 17 differentiation in a dichotomous relationship with Stat3.28 For this work, we began by examining baseline Stat5 phosphorylation in freshly isolated eGFP+ B6 Tregs. As seen in Figure 6D, naïve Tregs expressed phospho-Stat5 at low levels (left panel), which were modestly higher than a negative control (right panel). Next, we cultured WT or CCR8−/− eGFP+ B6 Tregs with eGFP– B6 DCs for 72 hours and examined the Tregs for the phosphorylated form of Stat5. As seen in Figure 6E, nearly all of the WT and CCR8−/− Tregs that survived the incubation period and retained their ability to produce eGFP expressed phospho-Stat5 at higher levels than naïve Tregs, suggesting that this transcription factor may be involved in the prosurvival signals transmitted to Tregs by DCs. The frequency of Tregs expressing phospho-Stat5 did not vary as a function of CCR8. However, a major difference in absolute Treg numbers was again noted between the 2 experimental groups despite exposure to identical B6 DC numbers (Figure 6F). Collectively, these results indicated that CCR8−/− Tregs display no qualitative differences in their ability to activate Stat5 antiapoptotic machinery compared with WT cells but appear to demonstrate a quantitative impairment in their ability to physically interact with survival-promoting antigen-presenting cells (APCs) over time.
If indeed CCR8 promotes Treg survival by promoting critical interactions with neighboring DCs, we reasoned that the survival defect displayed by CCR8−/− Tregs compared with WT Tregs would be minimized in a scenario where the Tregs would be unable to establish direct contact with APCs. To definitively test this hypothesis, we made use of transwell plates. As seen in Figure 6G-H, no differences in WT or CCR8−/− Treg numbers were noted when the cells were physically separated from activated B6 DCs or when no DCs were present. However, when both the Tregs and DCs were cultured together and therefore capable of direct cellular contact, greater numbers of WT Tregs were once again noted (Figure 6I). In summary, our in vitro studies are consistent with a mechanism in which the expression of CCR8 by Tregs enhances their survival by promoting physical interactions with DCs.
Donor APCs are critical for donor Treg maintenance after transplant, but host APCs are dispensable for early Treg expansion
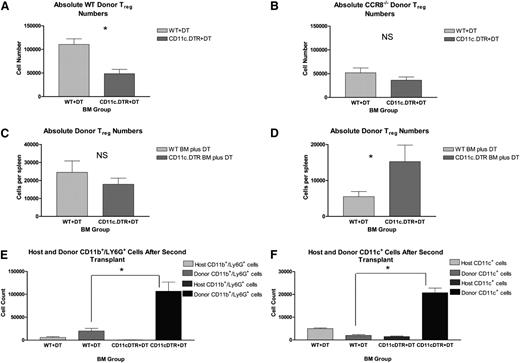
Consistent with previous reports, our data indicated that syngeneic DCs are able to promote Treg survival.25 As a consequence, we hypothesized that in the HSCT setting, donor APCs derived from the donor BM play a role in maintaining donor Tregs posttransplant and that their depletion could recapitulate the transplant results observed with CCR8−/− Tregs. To test this hypothesis, we performed a series of experiments using B6 mice transgenic for eGFP and the diphtheria toxin receptor (DTR) under control of the CD11c promoter, a cell surface marker expressed by APCs including classic and plasmacytoid DCs (“CD11c-DTR/eGFP” B6 mice). In brief, irradiated B6D2 recipients were transplanted with TCD BM from either WT B6 or CD11c-DTR/eGFP B6 mice along with WT Thy1.1+ B6 Tregs and Thy1.2+ B6 Tcons. All recipient mice were then administered diphtheria toxin (DT) by intraperitoneal injection. In this situation, CD11c-expressing APCs derived from the donor BM in those animals receiving CD11c-DTR/eGFP B6 BM but not WT B6 BM were selectively eliminated by the administration of DT. On transplant day +12, donor Tregs were quantified within recipient spleens using flow cytometry. Because only those donor Tregs arising from the initial Treg inoculum were congenic for Thy1.1, we were able to distinguish these cells from any new Foxp3+ cells that may have arisen from the Thy1.2+ donor BM.
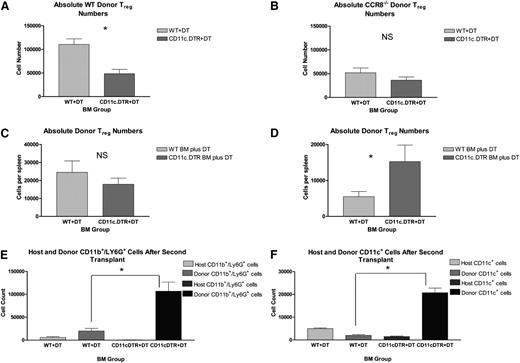
Across experiments, we were able to consistently deplete donor (Kd–) CD11c+ cell numbers by 70% to 80% in those mice given CD11c-DTR/eGFP BM compared with those receiving WT BM cells (supplemental Figure 5). As seen in Figure 7A, this reduction in donor APCs resulted in a 60% drop in Thy1.1+ donor Tregs in the recipient spleen by transplant day +12, suggesting that donor BM-derived APCs do contribute to the continued maintenance of donor Tregs after transplant.
Critical role for donor CD11c-expressing cells in the persistence of donor Tregs in vivo. (A) B6D2 recipients were lethally irradiated on day −1 and then administered 3 × 106 TCD BM cells from either WT Thy1.2+ B6 or CD11c-DTR/eGFP Thy1.2+ B6 mice plus 1 × 106 CD4+CD25+ WT Thy1.1+ B6 Tregs on day 0. Then 4 × 106 CD25-depleted Thy1.2+ Tcons from WT B6 donors were administered on day +2. Recipient mice were treated with DT via intraperitoneal injection at a dose of 4 ng/g body weight on transplant days +6, +8, and +10. On transplant day +12, recipient mice were euthanized, and their spleens disrupted. Donor Treg numbers were then quantified by flow cytometry using a Thy1.1+Foxp3+ gate; n = 6 WT BM and 7 CD11c-DTR/eGFP BM recipients. Absolute donor Treg numbers within the recipient spleen are depicted. *P = .0014 by Student t test. (B) B6D2 recipients were lethally irradiated on day −1 and then administered 3 × 106 TCD BM cells from either WT B6 or CD11c-DTR/eGFP B6 mice plus 1 × 106 CD4+CD25+ CCR8−/− eGFP+ B6 Tregs on day 0. Then 4 × 106 CD25-depleted Tcons from WT B6 donors were administered on day +2. Recipient mice were treated with DT via intraperitoneal injection on transplant days +6, +8, and +10. On transplant day +12, recipient mice were euthanized, and their spleens disrupted. Donor Treg numbers were then quantified by flow cytometry using an eGFP+Foxp3+ gate; n = 6 WT BM and 7 CD11c-DTR/eGFP BM recipients. P = .213. (C-D) “WT” BALB/c and “CD11c.DTR” BALB/c BM chimeras were generated and then used as secondary transplant recipients 8 to 12 weeks later. For these second transplants, WT and CD11c.DTR BALB/c BM chimeric mice were irradiated to 800 rads on transplant day −1. Recipient mice were administered 5 × 106 TCD BM cells from Thy1.1+ WT B6 mice and 5 × 105 Thy1.2+ WT B6 Tregs on transplant day 0. These cells were administered with (C) and without (D) 2.5 × 105 whole Tcons on day +2. All mice received DT by intraperitoneal injection on transplant days −3, −1, and +1. In the figure label, the “BM Group” refers to the BM product used to generate the 2 varieties of chimeric mice. Both groups of recipients received identical marrow products for transplant 2. On transplant day +7, recipients were euthanized, and their spleens disrupted. Donor Treg numbers within the recipient spleen were then quantified by flow cytometry using a Thy1.1–Kd–Foxp3+ gate. (C) n = 6 WT BM chimera mice and 7 CD11c.DTR BM chimeras. P = .381 by Student t test. (D) n = 7 WT BM chimera mice and 5 CD11c.DTR BM chimeras. *P = .041. (E-F) “WT” BALB/c and “CD11c.DTR” BALB/c BM chimeras were generated and then used as secondary transplant recipients 12 weeks later. For these second transplants, WT and CD11c.DTR BALB/c BM chimeric mice were irradiated to 800 rads on transplant day −1 and administered 5 × 106 TCD BM cells from Kb+ WT B6 mice without supplementary Tcons or Tregs on transplant day 0. All mice received DT by intraperitoneal injection on transplant days −3, −1, and +1. On transplant day +7, recipients were euthanized, and their spleens disrupted. (D) Donor neutrophil numbers within the recipient spleen were quantified by flow cytometry using a Kd–CD11b+Ly6G+ gate; n = 3 mice per group. *P = .014 by Student t test. (E) Donor CD11c+ APC numbers within the recipient spleen were quantified by flow cytometry using a Kd–CD11c+ gate; n = 3 mice per group. *P = .001.
Critical role for donor CD11c-expressing cells in the persistence of donor Tregs in vivo. (A) B6D2 recipients were lethally irradiated on day −1 and then administered 3 × 106 TCD BM cells from either WT Thy1.2+ B6 or CD11c-DTR/eGFP Thy1.2+ B6 mice plus 1 × 106 CD4+CD25+ WT Thy1.1+ B6 Tregs on day 0. Then 4 × 106 CD25-depleted Thy1.2+ Tcons from WT B6 donors were administered on day +2. Recipient mice were treated with DT via intraperitoneal injection at a dose of 4 ng/g body weight on transplant days +6, +8, and +10. On transplant day +12, recipient mice were euthanized, and their spleens disrupted. Donor Treg numbers were then quantified by flow cytometry using a Thy1.1+Foxp3+ gate; n = 6 WT BM and 7 CD11c-DTR/eGFP BM recipients. Absolute donor Treg numbers within the recipient spleen are depicted. *P = .0014 by Student t test. (B) B6D2 recipients were lethally irradiated on day −1 and then administered 3 × 106 TCD BM cells from either WT B6 or CD11c-DTR/eGFP B6 mice plus 1 × 106 CD4+CD25+ CCR8−/− eGFP+ B6 Tregs on day 0. Then 4 × 106 CD25-depleted Tcons from WT B6 donors were administered on day +2. Recipient mice were treated with DT via intraperitoneal injection on transplant days +6, +8, and +10. On transplant day +12, recipient mice were euthanized, and their spleens disrupted. Donor Treg numbers were then quantified by flow cytometry using an eGFP+Foxp3+ gate; n = 6 WT BM and 7 CD11c-DTR/eGFP BM recipients. P = .213. (C-D) “WT” BALB/c and “CD11c.DTR” BALB/c BM chimeras were generated and then used as secondary transplant recipients 8 to 12 weeks later. For these second transplants, WT and CD11c.DTR BALB/c BM chimeric mice were irradiated to 800 rads on transplant day −1. Recipient mice were administered 5 × 106 TCD BM cells from Thy1.1+ WT B6 mice and 5 × 105 Thy1.2+ WT B6 Tregs on transplant day 0. These cells were administered with (C) and without (D) 2.5 × 105 whole Tcons on day +2. All mice received DT by intraperitoneal injection on transplant days −3, −1, and +1. In the figure label, the “BM Group” refers to the BM product used to generate the 2 varieties of chimeric mice. Both groups of recipients received identical marrow products for transplant 2. On transplant day +7, recipients were euthanized, and their spleens disrupted. Donor Treg numbers within the recipient spleen were then quantified by flow cytometry using a Thy1.1–Kd–Foxp3+ gate. (C) n = 6 WT BM chimera mice and 7 CD11c.DTR BM chimeras. P = .381 by Student t test. (D) n = 7 WT BM chimera mice and 5 CD11c.DTR BM chimeras. *P = .041. (E-F) “WT” BALB/c and “CD11c.DTR” BALB/c BM chimeras were generated and then used as secondary transplant recipients 12 weeks later. For these second transplants, WT and CD11c.DTR BALB/c BM chimeric mice were irradiated to 800 rads on transplant day −1 and administered 5 × 106 TCD BM cells from Kb+ WT B6 mice without supplementary Tcons or Tregs on transplant day 0. All mice received DT by intraperitoneal injection on transplant days −3, −1, and +1. On transplant day +7, recipients were euthanized, and their spleens disrupted. (D) Donor neutrophil numbers within the recipient spleen were quantified by flow cytometry using a Kd–CD11b+Ly6G+ gate; n = 3 mice per group. *P = .014 by Student t test. (E) Donor CD11c+ APC numbers within the recipient spleen were quantified by flow cytometry using a Kd–CD11c+ gate; n = 3 mice per group. *P = .001.
Given our previous in vitro data suggesting a contribution by CCR8 to the ability of Tregs to colocalize to neighboring DCs, we reasoned that CCR8−/− donor Tregs might not be affected by donor APC depletion, because they would fail to efficiently migrate to donor DCs. To test this hypothesis, we performed a similar transplantation experiment but substituted eGFP+Thy1.2+CCR8−/− Tregs for WT Thy1.1+ Tregs. Consistent with our prior observations, donor CCR8−/− Tregs were observed in lower numbers than WT donor Tregs within recipient spleens on transplant day +12 in both WT and CD11c-DTR/eGFP BM recipients (Figure 7A-B). Also, as predicted, the depletion of donor DCs did not have a statistically significant impact on the number of donor CCR8−/− Tregs present. Collectively, these data indicated that donor DCs are much more critical for the maintenance of WT donor Tregs compared with CCR8−/− donor Tregs.
Next, we turned our attention to the contribution of host APCs to donor Treg expansion and survival. For this, we used a B6 into BALB/c transplant model system with either WT BALB/c or CD11c -DTR/eGFP BALB/c mice as our transplant recipients. In this scenario, host CD11c APCs can be depleted in the latter by the administration of DT. Unfortunately, DTR expression in commercially available CD11c-DTR/eGFP BALB/c mice is not restricted to the hematopoietic compartment, with DTR also appearing on pulmonary endothelial cells. As a result, CD11c-DTR/eGFP mice become extremely susceptible to DT toxicity following lethal irradiation. To circumvent this problem, we generated BALBc->BALB/c (“WT”) and CD11c-DTR/eGFP BALB/c-> BALB/c (“CD11c.DTR”) BM chimeras and then used these as secondary transplant recipients. For these second transplants, WT and CD11c.DTR chimeric mice were lethally irradiated and given DT by intraperitoneal injection. Recipient mice were administered TCD BM from Thy1.1+ B6 mice with Tcons and Tregs from Thy1.2+ WT B6 donors. Donor Treg numbers within the recipient spleen were then quantified by flow cytometry on day +7. As seen in Figure 7C, we found that the depletion of host CD11c+ APCs did not have a statistically significant effect on donor Treg numbers after transplant. In order to rule out any potentially confounding effects that host APC depletion might have on donor Tcon expansion and overall IL-2 production, we performed an identical transplant in the absence of supplemental donor Tcons. As seen in Figure 7D, we again failed to observe a reduction in donor Treg numbers in the setting of host APC depletion. Instead, donor Treg numbers were actually found to be enhanced. Taken together, these data indicated that host CD11c+ APCs are at least partially dispensable for early donor Treg engraftment and expansion after transplant.
Following the secondary transplantation of our BM chimera mice, we consistently observed varying degrees of splenomegaly in CD11c.DTR BALB/c chimeras following the administration of DT, as well as increased overall splenic cellularities compared with the WT BALB/c chimeras. As a result, we used flow cytometry to examine donor BM reconstitution in the setting of host CD11c+ APC depletion. Using a Kd– gate, we consistently found that both donor neutrophil and CD11c+ reconstitution were greatly enhanced in the setting of host CD11c+ APC depletion (Figure 7E-F, respectively). In contrast, the effects of host APC depletion on donor B-cell, natural killer–cell, and Tcon numbers were more variable, with these numbers increasing modestly in some but not all of our transplantation experiments (data not shown).
Discussion
In this study, we have used CCR8 knockout mice to examine the mechanisms by which donor Tregs prevent GVHD after transplant. Here, we show that extended Treg survival is crucial for long-term GVHD protection and that this in turn depends at least in part on functional CCR8. Previously, emphasis has been placed on the importance of the very early suppression of donor Tcon expansion within host secondary lymphoid tissue as the means by which Tregs prevent GVHD.6,7 Although these effects are likely important for GVHD attenuation, our findings indicate that they represent only a portion of the overall mechanism(s) by which donor Tregs are able to improve transplant outcomes. Here, we show that CCR8−/− Tregs ultimately fail to protect recipient mice against lethal GVHD despite demonstrating a normal capacity to accumulate within host secondary lymphoid tissue and a preserved ability to block early donor Tcon expansion.
It is well known that chemokine receptors contribute to lymphocyte homing in vivo. Our data, however, highlight their more subtle functional effects. CCR8−/− Tregs demonstrated in vivo trafficking patterns that were similar to WT cells. Despite this, Tregs lacking CCR8 were severely impaired in their ability to prevent GVHD because of an increased propensity to undergo cell death. To our knowledge, our data are the first to link a single chemokine receptor to Treg survival. The mechanisms by which CCR8 potentiates donor Treg survival in vivo are not entirely certain, although we suspect based on our in vitro data that CCR8 functions to direct donor Tregs toward donor DCs where they receive antiapoptotic signals. Although our data do not allow us to identify the specific DC subsets that mediate these effects in vivo, the prosurvival signals mediated by CCR8 appear to depend on cell contact because WT and CCR8−/− Tregs were observed in similar numbers after being incubated with syngeneic DCs in transwell plates. The downstream signaling events following DC engagement are incompletely understood. However, we found that Tregs strongly phosphorylated Stat5 after coculture with syngeneic DCs, a protein that is known to mediate antiapoptotic effects in regulatory T cells. Notably, the in vitro stimulation of CCR8 with either of its known ligands was insufficient to directly alter Treg survival and/or expansion. Furthermore, CCR8−/− Tregs did not demonstrate any impairment in their ability to respond to soluble IL-2, as WT and CCR8−/− persisted to a similar degree when cultured with saturating amounts of IL-2 in the absence of antigen presenting cells.
As described previously, CCR8’s prosurvival properties depended on the presence of DCs in vitro. In our transplantation experiments, however, we discovered that host- and donor-derived CD11c+ APCs produce substantially different effects on donor Tregs. As anticipated, donor BM-derived APCs played a role in maintaining the initial donor Treg inoculum. Unexpectedly, however, the depletion of host BM-derived APCs failed to reduce donor Treg numbers and even increased them in some experiments. Because we were not able to completely deplete all host CD11c+ cells, we cannot conclusively state that host CD11c+ APCs play no role in donor Treg activation. However, our data would indicate that this population of cells is at least partially dispensable for early Treg expansion.
Although initially unexpected, our APC depletion data are consistent with a previous report. In work by Tawara et al,29 the authors examined the influence of host APCs on the expansion and maintenance of donor Tregs posttransplant using host animals unable to express class II MHC. There, class II MHC expression by recipient hematopoietic cells appeared to be required for Treg-mediated GVHD protection but was dispensable for donor Treg proliferation and maintenance. When donor BM-derived APCs were unable to express class II MHC, however, a significant drop in donor Treg numbers was observed. Our own DTR chimeric transplants varied from the model system employed previously. However, our data would support their finding that donor APCs are primarily responsible for supporting donor Treg numbers after transplant.
In several experiments, we observed splenomegaly and an increase in myeloid reconstitution following the administration of DT to CD11c.DTR chimeric mice. In previous reports, neutrophilia and monocytosis have been reported following the administration of DT to nontransplanted CD11c-DTR/eGFP mice.30-32 In those studies, the increased myeloid numbers were attributed to an accelerated release of neutrophils from the BM in response to CXC chemokine ligand 1 and/or 2, and to enhanced granulopoiesis. Whether the increased myeloid engraftment observed in our CD11c.DTR chimeric recipients was a direct effect of the DT itself or secondary to the enhanced depletion of host APCs that occurs in these mice is uncertain. Notably, no neutrophilia was detected following the administration of DT to WT mice receiving donor CD11c.DTR BM cells, suggesting that host APCs in particular may exert myelosuppressive effects early after stem cell transplantation.
From a translational standpoint, our Treg data suggest that the CCR8 signaling axis could be targeted as a means for manipulating natural Treg numbers in vivo. Although Tregs can clearly produce beneficial effects in the HSCT setting, Tregs have been linked to a breakdown of antitumor immunity.33-40 Therefore, it may at times be desirable to deplete Treg numbers in vivo, a task that is often difficult with anti-CD25 antibody and/or cytotoxic chemotherapy.41 The chemical structures for small-molecule antagonists against human CCR8 have already been published42 and could form the basis for future drug development. Furthermore, a previous study demonstrated the potential feasibility of this approach, with neutralizing antibodies against CCL1 leading to reduced numbers of intratumor Tregs and solid tumor regression in a murine breast cancer model.43 In the hematologic malignancy setting, the inhibition of CCR8 signaling might be pursued as a means for boosting antileukemia immunity following autologous transplant or during standard acute myeloid leukemia induction.
In conclusion, we have demonstrated a critical role for CCR8 in the maintenance of donor Tregs after transplant. In so doing, we have identified a potential drug target for the manipulation of Treg numbers in vivo and have shed new light on the contributions of both donor- and host-derived APCs to donor Treg function after HSCT.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the following National Institutes of Health grants: National Cancer Institute 5K12CA120780-05 and National Heart, Lung, and Blood Institute 5K08HL111205-02 (J.M.C.); and National Heart, Lung, and Blood Institute R01HL115761, National Cancer Institute RO1CA166794, and National Institute of Allergy and Infectious Diseases R56 AI064363 (J.S.S.).
Authorship
Contribution: J.M.C. performed experiments and wrote the manuscript; K.A.F., M.L.W., L.M.F., B.G.V., K.L., A.P.-M., K.P.M., and H.v.D. performed, designed, or interpreted experiments; D.N.C. derived the CCR8−/− mice and reviewed the manuscript; B.R.B. reviewed the manuscript; and J.S.S. conceived the project and assisted in the writing of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: James M. Coghill, Lineberger Comprehensive Cancer Center, 450 West Dr, The University of North Carolina at Chapel Hill, Chapel Hill, NC 27599-7305; e-mail: jcoghill@e-mail.unc.edu.

![Figure 2. Absence of CCR8 does not impair Treg activation or suppressive function. (A-B) B6D2 mice were lethally irradiated on day −1 and then transplanted with 3 × 106 WT B6 TCD BM cells with either 1 × 106 WT eGFP+ B6 or CCR8−/− eGFP+ B6 Tregs on day 0. Then 4 × 106 WT eGFP– B6 Tcons were administered on day +2. On transplant day +7, recipient mice were euthanized, and their spleens disrupted. Donor Tregs were then examined for activation markers using a Foxp3+eGFP+ gate. Representative flow cytometry plots following l-selectin (A) and CD44 (B) staining are depicted. In addition, the mean percentages of l-selectinlow and CD44high cells are depicted graphically; n = 9 total recipients per group. The spleens were pooled into groups of 3 to maximize the number of donor events, and mean percentages of the groups compared using the Mann-Whitney Test. (C) 1.5 × 106 freshly isolated CD4+CD25+ WT or CCR8−/− Tregs were labeled with CFSE and transplanted with 3 × 106 unlabeled WT B6 TCD BM cells into irradiated B6D2 recipients. On transplant day +5, recipient mice were euthanized, their spleens disrupted, and donor Tregs examined for CFSE positivity by flow cytometry using a Kd–, Foxp3+ gate. (D) CD4+CD25+ Tregs were isolated from WT or CCR8−/− B6 mice and then cultured for 72 hours at varying ratios with 5 × 104 CD4+CD25– WT B6 responder cells and 5 × 104 irradiated TCD B6D2 stimulator splenocytes. During the last 16 to 20 hours of incubation, 0.037 MBq (1 µCi) of [3H]thymidine was added to each well, and [3H]thymidine incorporation measured by scintillation counting.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/122/5/10.1182_blood-2012-06-435735/4/m_825f2.jpeg?Expires=1769086648&Signature=j4E46gpUvew17xoZoPAAwStzDgIMXvVV76b9ZWRIiBKPSqQ0MCsvucg-13IiDX62cuj4eIFy0V3XzZ1MA9N17wrno8s3fRMRwaYey7dPkf6o9xEF-Ss3HUvMj4qYR9mgHg7ZN-AbuKFHGUC4unTT7qJk3gkLDYgJz9h0zGtuGXtHmNGcQUF2d26Du4qbO1AYlLpZ~UMwuwyd87cLwXGBjwQHx3YM2dbQ9s3RscFFKx-joFXED0jzBja~w1vSBSMfllN08DWB1sKecrkPT7abgEqfQpEABrK3bkZCOalU01aHNqhhoaCNGuxNj6Z3GJe6MCob4h97vcKzM5THLeQTOw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


![Figure 2. Absence of CCR8 does not impair Treg activation or suppressive function. (A-B) B6D2 mice were lethally irradiated on day −1 and then transplanted with 3 × 106 WT B6 TCD BM cells with either 1 × 106 WT eGFP+ B6 or CCR8−/− eGFP+ B6 Tregs on day 0. Then 4 × 106 WT eGFP– B6 Tcons were administered on day +2. On transplant day +7, recipient mice were euthanized, and their spleens disrupted. Donor Tregs were then examined for activation markers using a Foxp3+eGFP+ gate. Representative flow cytometry plots following l-selectin (A) and CD44 (B) staining are depicted. In addition, the mean percentages of l-selectinlow and CD44high cells are depicted graphically; n = 9 total recipients per group. The spleens were pooled into groups of 3 to maximize the number of donor events, and mean percentages of the groups compared using the Mann-Whitney Test. (C) 1.5 × 106 freshly isolated CD4+CD25+ WT or CCR8−/− Tregs were labeled with CFSE and transplanted with 3 × 106 unlabeled WT B6 TCD BM cells into irradiated B6D2 recipients. On transplant day +5, recipient mice were euthanized, their spleens disrupted, and donor Tregs examined for CFSE positivity by flow cytometry using a Kd–, Foxp3+ gate. (D) CD4+CD25+ Tregs were isolated from WT or CCR8−/− B6 mice and then cultured for 72 hours at varying ratios with 5 × 104 CD4+CD25– WT B6 responder cells and 5 × 104 irradiated TCD B6D2 stimulator splenocytes. During the last 16 to 20 hours of incubation, 0.037 MBq (1 µCi) of [3H]thymidine was added to each well, and [3H]thymidine incorporation measured by scintillation counting.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/122/5/10.1182_blood-2012-06-435735/4/m_825f2.jpeg?Expires=1769086649&Signature=KF-3OObWj0N5c5a~1eKUqXFK-kusE1tLa9i9MrfQxyTpvPMMXzGcfo6ibAS0kk62v-~khsOQXCiPrCvFGHH8vOly3MuSSh7tFXDKxIgfD13M3JhIy~aKEM7ehKuCjN6GF6q-LPC7IsZRbZo6KPMMLWmrUVunA0VUwI8ixhTnXzff0SKW9eEuh-12Zw4YfrcDwP71Hr~8gxZ8MGis-k~uJcz0qve61Z7GALnJAqYXVigK9si7SB3mlw-8FQnJu7WEbKsb6UbG5oDhvp~xMRvUKQtyl38U0e9lw0PhL0aPCLSU54AqF2Tqxi9OSGZlI~wIYz6r5lvbQzAH7hG~oeGLUw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)