Key Points
Before HCT 61% of men and 37% of women were sexually active; the 3-year prevalence declined to 54% for men but increased to 52% for women.
Chronic GVHD in both sexes and TBI in men contribute to sexual dysfunction and dissatisfaction over the 3 years following HCT.
Abstract
This prospective study described the trajectory of sexual well-being from before hematopoietic cell transplantation (HCT) to 3 years after in 131 allogeneic and 146 autologous HCT recipients using Derogatis Interview for Sexual Function and Derogatis Global Sexual Satisfaction Index. Sixty-one percent of men and 37% of women were sexually active pre-HCT; the prevalence declined to 51% (P = .01) in men and increased to 48% (P = .02) in women at 3 years post-HCT. After HCT, sexual satisfaction declined in both sexes (P < .001). All sexual function domains were worse in women compared with men (P ≤ .001). Orgasm (P = .002) and drive/relationship (P < .001) declined in men, but sexual cognition/fantasy (P = .01) and sexual behavior/experience (P = .01) improved in women. Older age negatively impacted sexual function post-HCT in both sexes (P < .01). Chronic graft-versus-host disease was associated with lower sexual cognition/fantasy (P = .003) and orgasm (P = .006) in men and sexual arousal (P = .05) and sexual satisfaction (P = .005) in women. All male sexual function domains declined after total body irradiation (P < .05). This study identifies vulnerable subpopulations that could benefit from interventional strategies to improve sexual well-being.
Introduction
As survival continues to improve for a growing number of hematopoietic cell transplantation (HCT) recipients, quality of life (QOL) concerns assume increasing importance. Sexual well-being is an important component of QOL, yet there is limited information on the longitudinal trajectory of sexual well-being and the sociodemographic and clinical factors that modify this trajectory.1-3 To date, few longitudinal studies have examined sexual function in >50 HCT recipients with assessments performed prior to HCT and at time points extending beyond 1 year after HCT.4-6 Furthermore, these studies have included primarily non-Hispanic white patients (>70%) and allogeneic HCT recipients (>90%).
The current prospective longitudinal study addresses these gaps by determining sexual well-being before HCT and at predetermined time points up to 3 years after autologous and allogeneic HCT in a large, ethnically diverse population. We hypothesized that sexual well-being for both men and women would decline at 6 months after HCT, and then demonstrate varying levels of recovery that depended on age, sex, conditioning, donor source, and, among allogeneic HCT recipients, the presence of chronic graft-versus-host disease (GVHD).
Methods
Study participants
The institutional Human Subjects Protection Committee approved the protocol; written informed consent was obtained according to the Declaration of Helsinki. This report was part of a QOL study of English-speaking patients aged 18 years or older who underwent HCT at City of Hope for hematologic diseases between February 2001 and January 2005. In addition, to be eligible, the patients needed to be healthy enough and have sufficient time before HCT to consent and participate in the study.7 The Derogatis Interview for Sexual Function–Self Report (DISF-SR) and the Global Sexual Satisfaction Index (GSSI) of the Derogatis Sexual Functioning Inventory were used for assessing sexual function. The City of Hope Quality of Life (COH-QOL-HCT) questionnaire was used for assessing health-related QOL.7 All consented patients completed the questionnaires before HCT, and at 6 months, 1, 2, and 3 years after HCT. Follow-up phone calls were made 2 weeks after nonresponse and up to 4 months for the 6-month survey and up to 10 months for subsequent surveys. Nonresponse thereafter was considered refusal for that time point only.
A total of 609 eligible patients were approached to participate; of these, 299 refused to participate explicitly or passively. Of the 310 (51%) who consented and completed the pre-HCT QOL and/or sexual function surveys, 33 were excluded (4 completed the pre-HCT survey after HCT [n = 2] or more than 6 months before HCT [n = 2]; 29 did not respond to any sexual function surveys). Of the 277 with at least 1 sexual function survey completed, 21 did not respond at the pre-HCT time point but responded at 1 or more post-HCT time points and were retained in the analysis (supplemental Figure 1, available on the Blood Web site). DISF-SR was completed at least once by 236 patients and GSSI by 225 patients. Response rates averaged 85% across post-HCT time points.
Derogatis Sexual Functioning Inventory–GSSI
GSSI8 elicits respondents’ sexual satisfaction level at the time of survey using a scale ranging from 0 (“could not be worse”) to 8 (“could not be better”) (supplemental Table 1). GSSI was administered to participants regardless of their sexual activity at the time of study participation.
Derogatis Interview for Sexual Function–Self Report
Gender-specific DISF-SR8 (supplemental Tables 2-3) included 25 items evaluating 5 domains of sexual functioning in the 30 days before study participation, paralleling the 5 phases of sexual response cycle: (1) cognition/fantasy (5 items); (2) sexual arousal (5 items); (3) sexual behavior/experience (5 items); (4) orgasm (6 items); and (5) drive/relationship (4 items). Total sexual function score across all domains and domain-specific scores were computed. Higher scores indicated better sexual functioning. The frequency of sexual activity was derived from an item in the sexual behavior/experience domain. Psychometric properties of DISF-SR demonstrate internal consistency from 0.74 for drive/relationship to 0.80 for orgasm, and test-retest reliability (1-week interval) from 0.80 to 0.90.8
Demographic and clinical information
Age, gender, race/ethnicity, educational status, income, marital status, height, weight, and history of chronic GVHD were obtained via self-report. Missing data on education (23%) and income (43%) were imputed using the median education and income data from the 2000 US Census at the block level of patients’ residence at the time of transplantation. Primary diagnosis, conditioning regimens, stem cell source, risk of relapse at HCT, and disease status pre-HCT, and at 6 months, 1, 2, and 3 years after HCT were abstracted from medical records. Medication data were obtained from medical records, and supplemented by self-report.
Statistical analysis
Clinical and demographic characteristics were compared between study participants and nonparticipants using the t test and χ2 test. Multivariable logistic regression was used to identify predictors of nonparticipation. Overall survival was compared between participants and nonparticipants using the log-rank test.
Generalized estimating equation (GEE),9 using the compound symmetry working correlation matrix to account for within-person correlation, was used to model the time trends of sexual satisfaction, total sexual function, and sexual function domain scores, and to identify modifying factors. Analysis was stratified by gender. Square-root transformation was used to normalize the scores for sexual arousal and sexual behavior/experiences in men and sexual cognition/fantasy, sexual arousal, sexual behavior/experiences, and total sexual function in women. Time in weeks since transplantation was used in a piece-wise model connecting the pre-HCT to 6-month post-HCT time points, and a linear or quadratic function of time between the 6-month and 3-year post-HCT time points.
Time-invariant independent covariates included age at referent HCT, race/ethnicity (non-Hispanic white, other), annual household income before HCT (treated as a continuous variable using midpoints of the original categorical response: <$20 000, $20 000 to $49 999, $50 000 to $74 999, $75 000 to $100 000, >$100 000; using $10 000 for the lowest and $125 000 for the highest levels), highest education attained at HCT (treated as continuous variable using 9, 12, 14, 16, 19 years of education for the original categorical response of less than high school, high school, some college, bachelor’s degree, and post-graduate degree, respectively), primary diagnosis (acute leukemia [lymphoblastic (ALL) and myeloid (AML)]; lymphoma [Hodgkin (HL) and non-Hodgkin (NHL)]; multiple myeloma [MM]; other diagnoses [chronic lymphocytic leukemia (CLL), chronic myelogenous leukemia (CML), severe aplastic anemia (SAA), other-leukemia]), stem cell source (allogeneic, autologous), exposure to total body irradiation (TBI), number of HCTs (1, >1), and risk of relapse at HCT (standard, high). In the event of multiple HCTs, the transplant that prompted study enrollment was treated as the referent HCT. Patients transplanted in first or second complete remission after AML, ALL, HL, or NHL, first chronic phase of CML, or patients with SAA were considered to be at standard risk of relapse at HCT; all others were considered at high risk.
Time-varying independent covariates included marital status (married/not married), body mass index (BMI: kg/m2), change in BMI from pre-HCT level, chronic GVHD (present/absent), relapse of primary disease (yes/no), use of medications known to enhance/salvage sexual functions (hormone replacement therapy, medication prescribed to treat sexual dysfunction) (yes/no), and other medications known to decrease sexual function (antidepressants, cardiac medications) (yes/no). Covariate values concurrent with domain scores were used.
The relationships between sexual QOL and physical and psychological QOL were examined by including the summary scores for the respective domains at the corresponding time points in the GEE models for total sexual function.
Stepwise regression with P < .10 was used to select variables to enter in the following order: sociodemographic variables, clinical variables, and exposure to TBI and other conditioning agents. Variables with the 2-sided Wald test (P < .05) were retained in the final model. Time-specific effect size (ES) of a covariate was computed as the absolute difference between estimated scores of the index and referent groups divided by the common SD. The average ES across post-HCT time points and the percentage difference between groups were computed. Cohen’s definition of ES (small ES = 0.2, medium ES = 0.5, and large ES = 0.8) was used.10 Because an ES of one-third to one-half could be considered discriminatory for health-related QOL,11,12 we regarded ES ≥ 0.3 as clinically relevant.
PROCs LOGISTIC, LIFETEST, and GEE of SAS 9.2 (SAS Institute) were used.
Results
Participants were comparable to nonparticipants across all clinical and sociodemographic characteristics except education (participants were more likely to have some college education, P < .001; data not shown). After adjusting for sex, age, income, stem cell source, and diagnosis, there was no difference in survival between participants and nonparticipants (P = .63).
The median age of the cohort at HCT was 48 years; 55% were men; 66% were non-Hispanic white; 45% had a bachelor’s degree or higher; 36% had income of $75 000 or higher; 47% were allogeneic HCT recipients; and 45% received TBI (Table 1). Primary diagnoses included acute leukemia (36%), lymphoma (36%), MM (18%), and other diagnoses (9%). The pre-HCT survey was completed at a median of 17 days before HCT (range, 0-160; data not shown).
Sexual activity
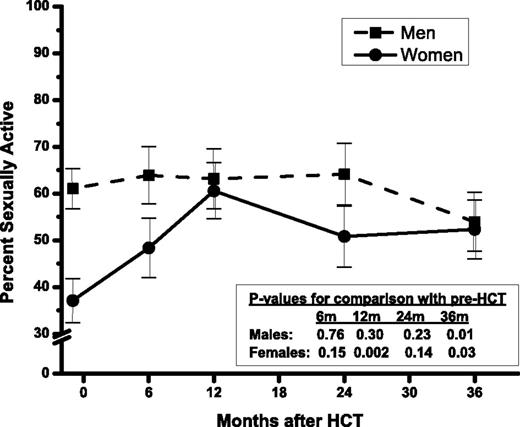
Prior to HCT, the prevalence of sexually active men and women (had sex with a partner at least once in the preceding month) was 61% and 37% (Figure 1), respectively (P < .001, adjusted for age, marital status). The prevalence of sexually active men remained stable for 2 years after HCT but decreased to 54% at 3 years (P = .01, compared with pre-HCT, adjusted for age, income, disease relapse). The reduction occurred primarily in autologous recipients (pre- vs 3 years post-HCT: 58% vs 45%, P = .02) and not in allogeneic recipients (65% vs 70%, P = .87). This could be explained in part by the increasing prevalence of relapsed patients over time among autologous HCT recipients (12%, 11%, 15%, 25% at 6 months, 1, 2, and 3 year post-HCT, respectively), when compared with allogeneic HCT recipients (12%, 5%, 6%, 8%). Disease relapse (described in the next paragraph) was associated with lower prevalence of sexually active male patients. On the other hand, the prevalence of sexually active women increased with time to 52% at 3 years (P = .03); this prevalence was similar to that for men (P = .68, adjusted for age and marital status). This increase may be explained by the corresponding improvement in psychological QOL post-HCT. There was no difference in the prevalence of sexually active women before and 3 years after HCT by stem cell source (autologous: 37% vs 55%; allogeneic: 37% vs 50%).
Percent ± SE of sexually active patients by time since HCT, in men and women.
Although men engaged in more frequent sexual activity than women before HCT (median of 1.5 vs 0 per month, P = .03), there was no difference after HCT (median of 1.5 for both sexes, P > .09 at all post-HCT time points). Older age (P = .002), lower income (P = .01), and disease relapse (P = .03) in men, and older age (P < .001), not being married (P < .001), and poorer physical QOL (P = .002) in women were associated with lower prevalence of sexually active individuals at all time points.
Sexual satisfaction
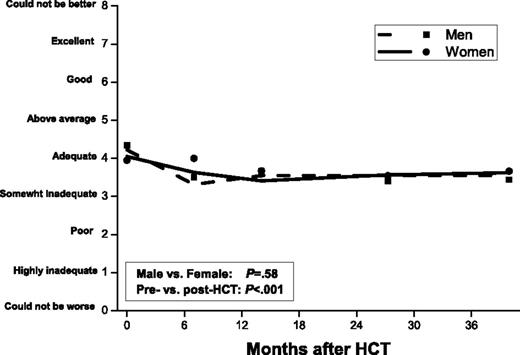
Adjusted for significant covariates, including sexual activity, men and women reported similar levels of sexual satisfaction before and up to 3 years after HCT (P = .58) (Figure 2). At the pre-HCT time point, sexual satisfaction score averaged 4.3 for men and 4.0 for women (4 = “adequate” on a scale of 0 “could not be worse” to 8 “could not be better”), declined to 3.5 at 6 months (3 = “somewhat adequate”; P < .001), and remained at this level to the 3-year post-HCT time point. Sexual satisfaction was higher for sexually active participants compared with sexually nonactive participants (pre-HCT mean ± SD, men: 5.0 ± 1.9 vs 3.0 ± 2.2, P < .0001; women: 5.5 ± 2.1 vs 2.8 ± 2.2, P < .0001; trends were similar after HCT).
Longitudinal trends of sexual satisfaction in men and women. Adjusted for age, race/ethnicity, income, change in BMI from pre-HCT level, and TBI.
Longitudinal trends of sexual satisfaction in men and women. Adjusted for age, race/ethnicity, income, change in BMI from pre-HCT level, and TBI.
Among men (Table 2), lower sexual satisfaction before HCT was associated with older age (P < .001), lower income (P = .02), and nonwhite race/ethnicity (P = .005). After HCT, lower sexual satisfaction was associated with older age at HCT (P < .001), a within-individual decline in BMI after HCT (P = .01), and exposure to TBI (P < .001). Among women (Table 3), lower sexual satisfaction after HCT was associated with receipt of allogeneic HCT (P = .02); this difference was attributed to chronic GVHD, as no difference was evident between autologous HCT recipients and allogeneic HCT recipients without chronic GVHD (P = .23). The mean sexual satisfaction score after HCT for female allogeneic HCT recipients with chronic GVHD was 2.9 (3 = “somewhat inadequate”) compared with 3.7 (4 = “adequate”) for autologous and allogeneic HCT recipients not reporting chronic GVHD (P = .005).
Sexual function
Sexual function scores were low compared with a normative community sample of 277 adults (49% men, mean age 34 years).13 Relative to the normative sample, the average percentile score over the study period for HCT recipients (adjusted to age 34 years, both sexes combined) was 16th for total sexual function, 40th for sexual cognition/fantasy, 23rd for sexual arousal, 23rd for sexual behavior/experiences, 12th for orgasm, and 23rd for drive/relationship (see supplemental Figure 2 for comparison of sexual function scores with normative sample).
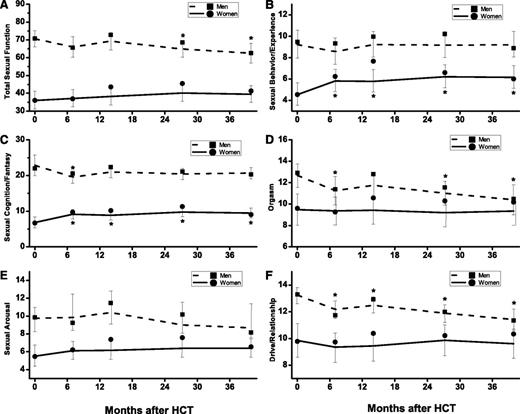
Sexual function was worse in women than in men across all domains (P ≤ .001). Longitudinal trends of sexual function are shown in Figure 3 and significant modifiers are summarized in Tables 2 and 3.
GEE estimates of the longitudinal trends of sexual function domains and total sexual function. (A) Total sexual function. (B) Sexual behavior/experience. (C) Sexual cognition/fantasy. (D) Orgasm. (E) Sexual arousal. (F) Drive/relationship. Men: dashed lines denote estimated function; ▪, observed mean. Women: solid lines denote estimated function; ●, observed mean. Vertical lines show 95% CIs of the GEE trends. *Significant (P < .05) difference from pre-HCT level.
GEE estimates of the longitudinal trends of sexual function domains and total sexual function. (A) Total sexual function. (B) Sexual behavior/experience. (C) Sexual cognition/fantasy. (D) Orgasm. (E) Sexual arousal. (F) Drive/relationship. Men: dashed lines denote estimated function; ▪, observed mean. Women: solid lines denote estimated function; ●, observed mean. Vertical lines show 95% CIs of the GEE trends. *Significant (P < .05) difference from pre-HCT level.
Sexual function among men
Total sexual function.
No clinically relevant temporal changes were observed. Worse sexual function before HCT was associated with older age (P < .001), lower pre-HCT BMI (P = .04), being married (P = .05), and lower education (P = .04). At all post-HCT time points, lower sexual function was associated with older age (P < .001), lower pre-HCT BMI (P = .04), post-HCT decline in BMI (P = .001), and exposure to TBI (P = .003). Total sexual function improved as physical QOL improved, both before (P = .002) and after HCT (P = .02). Sexual function also improved with psychological QOL post-HCT (P = .03); no association was identified prior to HCT (P = .20).
Sexual function domains.
Scores for orgasm (P = .002) and drive/relationship (P < .001) declined with time, reaching a clinically relevant reduction at 3 years post-HCT (ES = 0.36, 0.42, respectively) compared with the pre-HCT time point. Before HCT, lower scores were associated with older age (all domains, P < .001), being married (sexual arousal, P < .001), and lower education (sexual cognition/fantasy, P = .001). At all post-HCT time points, lower scores were associated with older age at HCT (all domains, P < .001), being married (sexual cognition/fantasy, P = .048; sexual arousal, P < .001), post-HCT decline in BMI (sexual cognition/fantasy, P = .02; sexual behavior/experience, P=.04; orgasm, P = .002; drive/relationship, P = .002), exposure to TBI (sexual cognition/fantasy, P = .04; sexual arousal, P = .07; sexual behavior/experience, P = .01; orgasm, P = .001; drive/relationship, P = .0003), and allogeneic HCT (sexual cognition/fantasy, P = .02; orgasm, P = .001). The effect of stem cell source was attributed to chronic GVHD; no difference was evident between autologous HCT recipients and allogeneic HCT recipients without chronic GVHD (sexual cognition/fantasy, P = .48; orgasm, P = .34). Allogeneic HCT recipients reporting chronic GVHD averaged 21% lower for sexual cognition/fantasy scores (ES = 0.42, P = .003) and 24% lower for orgasm scores (ES = 0.43, P = .006) after HCT compared with the scores for allogeneic HCT recipients without chronic GVHD combined with autologous HCT recipients.
Sexual function among women
Total sexual function.
No clinically relevant temporal changes were observed. Worse sexual function before HCT was associated with older age (P = .01) and high risk of relapse (P = .01). After HCT, worse sexual function was associated with lower income (P = .007). Total sexual function improved as physical QOL improved, both pre-HCT (P = .005) and post-HCT (P < .001). Sexual function also improved with psychological QOL post-HCT (P < .001) but no association was identified prior to HCT (P = .08).
Sexual function domains.
Scores for sexual cognition/fantasy (P = .01) and sexual behavior/relationship (P = .01) improved with time, reaching a clinically relevant increase at 2 years post-HCT (ES = 0.32 for both domains) compared with the pre-HCT time point. Before HCT, lower scores were associated with older age (sexual cognition/fantasy, P < .001; sexual arousal, P =.04; sexual behavior/experience, P =.005; drive/relationship, P = .001), being unmarried (sexual behavior/experience, P =.002; drive/relationship, P = .04), lower education (sexual cognition/fantasy, P = .001), high risk of relapse (sexual arousal, P = .007; drive/relationship, P = .03). At all post-HCT time points, lower scores were associated with older age at HCT (sexual cognition/fantasy, P < .001; sexual arousal, P =.001; sexual behavior/experience, P =.001; drive/relationship, P =.003), being unmarried (sexual behavior/experience, P =.008), being married (sexual cognition/fantasy, P = .004), lower education (sexual cognition/fantasy, P = .002; sexual arousal, P =.02), lower income (sexual cognition/fantasy, P = .04; drive/relationship, P =.002), non-Hispanic white race (sexual behavior/experience, P = .04), and chronic GVHD (sexual arousal, P = .05), but not stem cell source (P = .11).
Discussion
This prospective longitudinal study documents trends in sexual activity, satisfaction, and function in autologous and allogeneic HCT recipients. Prior to HCT, two-thirds of the male participants and 37% of the female participants were sexually active with a partner over the month prior to study participation. At 3-years post-HCT, 54% of men and 52% of women were sexually active. Seventy-six percent of the male and 49% of the female HCT recipients reported sex with or without a partner; these rates are similar to those reported by Syrjala et al in HCT recipients.6 Comparison of the rates in HCT recipients with national averages (89% for men and 74% women aged 25-64 years) is difficult because the latter rates are based on self-reported rates over a 6-month period prior to survey.14
Adjusted for sexual activity, both men and women on average reported “adequate” sexual satisfaction before HCT, and then experienced a significant decline, reporting “somewhat adequate” sexual satisfaction at 6 months, with no recovery at 3 years. Sexual satisfaction reported by the female HCT recipients was lower at all time points compared with healthy women of similar socioeconomic backgrounds.15 No comparable data using the same instrument were found for healthy men.
Sexual function scores were also low compared with a normative sample before and after HCT, which is consistent with previous studies that compared HCT patients to population norms,16 noncancer controls,17 cancer patients treated with conventional therapy,18 and healthy controls at 5 years after HCT.6 As in the general population19 and among HCT recipients,4-6,18,20 all sexual function domains were significantly worse in women compared with men. Whereas men experienced a decline over time in the orgasm and drive/relationship domains of sexual function, women experienced an improvement in sexual cognition/fantasy and sexual behavior/experience. Sexual satisfaction and function were worse at older age, as seen in other studies.4,5,18,21
Sexual satisfaction and function were better for those with higher education and annual household income, consistent with a previous study examining this issue in HCT recipients.22 In the general population, lower education is correlated with lack of sexual desire, erectile dysfunction, and premature ejaculation in men.23-25 Women of lower socioeconomic status experienced orgasm and sexual activity less frequently, had declining interest in sex, and more sexual problems.26-28 Differences in sexual education, awareness, and attitudes by social class were suggested as explanations.26
Lower sexual satisfaction and function were associated with a within-individual decline in BMI after HCT in men. Male patients with higher BMI also had better physical and psychological QOL post-HCT, and a larger decline in BMI after HCT was associated with lower psychological and social QOL,7 mirroring the trends for sexual satisfaction and functioning. Fatigue is correlated with poorer sexual functioning,16,18 which could partially explain our finding because fatigue was significantly worse in men who experienced greater weight loss, but not in women (data not shown).
Male allogeneic HCT recipients reported significantly fewer sexual thoughts or fantasies and were less satisfied with their orgasmic quality than autologous HCT recipients. Female allogeneic HCT recipients also reported lower sexual satisfaction. While a previous cross-sectional study also demonstrated worse sexual function among allogeneic HCT recipients compared with autologous HCT recipients,18 others failed to find such a relationship.29,30 Chronic GVHD explained the poorer sexual function in our allogeneic HCT recipients. Our previous QOL study also showed poorer QOL for those reporting chronic GVHD, which could be related to the adversely impacted sexual QOL.7 Syrjala et al reported that women (and not men) with extensive chronic GVHD had arousal problems at 3 years post-HCT.6 Another study found a significant correlation between chronic GVHD and sexual dysfunction only among men.21 Chronic GVHD in women can result in dyspareunia, vaginal irritation, and increased sensitivity of genital tissues.31 Although data on the location and extent of the chronic GVHD were not available, item-specific analysis (data not shown) showed that women with chronic GVHD reported significantly more problems with lubrication during sexual relations (P = .02) and masturbation (P = .04). Chronic GVHD in men can lead to inflammation of the genitals, scarring or adhesions in the blood vessels, and libido and erectile dysfunction,2,32 which likely contributed to the lower sexual function in our male patients.
Sexual satisfaction and all domains of sexual function were negatively impacted by exposure to TBI in men, but not in women. TBI is known to induce gonadal damage,33-35 yet previous studies have not reported an association between TBI and patient-reported sexual dysfunction in men.4,21,30 TBI can contribute to male genital tissue sensitivity, scarring, or atrophy.2 TBI is implicated in cavernosal arterial insufficiency, indicating vascular damage to corpora cavernosal vessels.32 Prospective studies of prostate cancer patients found erectile dysfunction after radiotherapy,36 which was attributed to vascular damage from irradiation.37,38 Among women, gonadal failure after exposure to TBI is well known,39,40 yet exposure to TBI was not associated with sexual dysfunction in the current study, even after adjusting for hormone replacement. Thus, we conjecture that differential effects of TBI may be due to inherent physiologic differences in the pathogenesis of sexual dysfunction among men and women.
This large prospective longitudinal study of sexual function reported by ethnically diverse allogeneic and autologous HCT recipients with determination of sexual function before and up to 3 years after HCT overcomes some of the limitations in previous studies. The three other longitudinal studies that assessed sexual function beyond 1 year after HCT had <100 patients or included predominantly allogeneic transplant recipients that were non-Hispanic whites.4-6 The study is also strengthened by the relatively low attrition and detailed domain-specific assessment of sexual function using validated, psychometrically sound instruments. Furthermore, the current study is the first to conclusively demonstrate the negative impact of chronic GVHD on specific domains of sexual functioning among both men and women. Finally, while the negative consequences of irradiation on erectile dysfunction are known in prostate cancer patients, the current study demonstrates for the first time the adverse consequence of TBI across all domains of sexual function in male HCT recipients.
Our study results should be interpreted in light of some limitations. We used census data to estimate missing data on patients’ education and income, with a potential risk of attenuating the effects of socioeconomic status41 ; however, aggregated census data were shown to be a reasonable proxy for patient-level socioeconomic status data in health outcome research.42,43 The lower than desired participation rate (51%) could possibly be due to reluctance to report on sexual functioning. However, no differences between participants and nonparticipants were found in sociodemographic/clinical characteristics or survival rates, suggesting that the study population was representative of the overall HCT population. Pre-HCT treatment data were unavailable, thus we could not tease out their effects from those of transplant-related conditioning. The lack of contemporaneous controls precludes comparison of the magnitude of sexual impairment in our HCT patients to non-HCT populations. However, comparison with historical normative samples indicates worse sexual functioning in transplant patients.
The efficacy of hormone replacement therapy, sildenafil for erectile dysfunction in men, and testosterone patch in women,44-46 has not been demonstrated in HCT patients. Medical treatment of the underlying physiological contributors to sexual dysfunction may be insufficient to fully ameliorate the problem because sexual dysfunction may also involve psychosocial causes.6,21,47 We demonstrated a positive association between sexual function and physical and psychological QOL in the current study. Increasing awareness of health care professionals about sexual dysfunction after HCT is also needed.48 Fewer than half of health care providers discuss sexuality issues other than fertility after HCT5 ; and, when queried, almost 20% of the patients are dissatisfied with the quality/quantity of information received regarding sexual sequelae of HCT.39
In conclusion, we found that nearly 50% of HCT survivors are sexually inactive at 3 years after HCT. The study confirms the persistent decline in sexual satisfaction in both men and women after HCT, and the significantly worse sexual functioning reported by female HCT recipients compared with their male counterparts for all domains. The study identifies diminished sexual function among older men and men exposed to TBI. The study also identifies the negative impact of chronic GVHD on sexual satisfaction among women and several domains of sexual function in both men and women. These findings demonstrate the need to develop effective communications between the transplant team and HCT survivors in order to identify concerns related to sexual dysfunction and address them in specialized multidisciplinary settings.
Presented in abstract form at the 49th annual meeting of the American Society of Hematology, San Francisco, CA, December 8-10, 2008.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Tracy Gould, a summer intern at City of Hope, for assistance in abstracting medication data from the patient medical records.
This work was supported in part by the National Cancer Institute at the National Institutes of Health P01 CA 30206 (S.J.F.), and Leukemia & Lymphoma Society Scholar Award for Clinical Research 2191-02 (S.B.)
Authorship
Contribution: F.L.W. performed the statistical analysis and wrote the paper; L.F., A.B., L.A., and C.H. performed data collection; K.T. and H.K. assisted in statistical analysis; M.G. and F.K. contributed to critical review of the paper; S.J.F. contributed to study design and critical review of the paper; and S.B. contributed to study conception, design, and analysis, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Smita Bhatia, Department of Population Sciences, City of Hope, 1500 E Duarte Rd, Duarte, CA 91010; e-mail: sbhatia@coh.org.