Key Points
Coagulation factor XII is not activated by platelets.
Abstract
The recent claim that stimulated platelets activate the intrinsic pathway of coagulation by the release of polyphosphates has been considered a breakthrough in hemostasis research. In little more than 3 years, the original publication by Müller et al has been cited >100 times. However, none of the citing articles has sought to independently validate this potentially paradigm-shifting concept. To this end, we performed extensive experimentation in vitro and in vivo in an attempt to verify the claim that factor XII (FXII) is primarily activated by stimulated platelets. In contrast to the original assertion, platelet-derived polyphosphates were found to be weak activators of FXII, with a FXIIa-generating activity of <10% compared with equivalent concentrations of kaolin. Using different coagulation assays, it was shown that platelet contribution to whole blood coagulation was unrelated to the generation of activated FXII in vitro. Additionally, key results used to verify the hypothesis in the original study in vivo were found to be irreproducible. We conclude that platelet-derived polyphosphates are not physiologically relevant activators of FXII.
Introduction
The enigmatic role of the intrinsic pathway of coagulation has been studied with renewed interest ever since it was shown that 1 strain of factor XII (FXII)-deficient mice are protected from thrombosis in various in vivo models, eg, in ferric chloride–induced thrombosis in mesenteric vessels, carotid vessels and inferior vena cava, and collagen-induced pulmonary embolism,1-3 and that FXII inhibitors protect from arterial thrombosis in various animal models.4-6 These studies provide strong evidence for a role for FXII in pathological thrombosis in mice, although clinical evidence for an analogous role in humans is still lacking.
One central question that has yet to be conclusively answered in this context is how FXII is activated during thrombosis in vivo.7 Several activators of FXII have been proposed, most notably fibrin clot surfaces,8 collagen exposed by damaged endothelium,9 misfolded or denatured proteins,10 and neutrophil extracellular traps.11 However, none of these potential activators have been validated conclusively in vivo.
Recently, Müller et al12 proposed that FXII activation in vivo is caused by negatively charged polyphosphates (polyP) secreted from platelet dense granules on platelet activation (hereinafter designated paper A). In a very recent review in Blood elaborating on these findings,13 one of the authors of paper A asserts that “the identification of polyP as a platelet-derived procoagulant agent provides a long-sought link between primary and secondary hemostasis and may represent a new paradigm for the treatment of thromboembolic and inflammatory diseases.” As the implications of this claim are considerable, it is imperative to independently verify the experimental results.
In the present study, we tested the following claims made in paper A: (1) platelet-derived polyphosphates are strong activators of FXII, equipotent with kaolin; (2) stimulated platelets activate physiologically relevant amounts of FXII; and (3) platelet contribution to whole blood coagulation is FXII dependent. As our results strongly indicated that these findings were incorrect, we proceeded to an assessment of the following results reported in paper A to verify the hypothesis in vivo: (4) that infusion of protease activated receptor 1 (PAR1) activating peptide Trap6 resulted in death by pulmonary embolism in 13 of 15 mice but that infusion of a phosphatase that degrades polyphosphate bonds salvaged challenged mice; and (5) that infusion of polyphosphate resulted in death by pulmonary embolism in 14 of 15 mice. The result reported in (4) was unexpected, because PAR1, the receptor activated by Trap6, is not known to be expressed by murine platelets.14-16 To exclude the possible influence of other mechanisms leading to the unexpected death of mice after challenge with Trap6, such as hypotension caused by activation of endothelial cells expressing PAR1,17 we reproduced the experiments in paper A but found no evidence of thrombotic events in challenged mice, even at concentrations of Trap6 that were 16 times higher than those used in paper A. When attempting to reproduce the experiments used to verify claim (5), we found that the polyP preparation used in paper A was not possible to dissolve at concentrations that would permit infusion of the stated amounts of polyP in challenged mice. Collectively, our results do not support the claim that platelets activate FXII by the release of polyphosphates.
Methods
Materials
Fluorogenic FXIIa substrate Boc-Gln-Gly-Arg-AMC and thrombin substrate Z-Gln-Gln-Arg-AMC were from Bachem (Bubendorf, Switzerland). Innovin, tissue factor (TF) with phospholipids, was from Dade Behring (Liederbach, Germany). Corn trypsin inhibitor (CTI) was from Hematologic Technologies (Essex Junction, VT). Recombinant chicken annexin V was from November (Erlangen, Germany). PAR1 activating peptide (Trap6) and PAR4 activating peptide (PAR4-AP) were from JPT Peptide Technologies (Berlin, Germany). The collagen-related peptide (CRP) was kindly provided by Dr R. W. Farndale and Dr G. Knight (Cambridge, UK) and was cross-linked with N-succinimidyl 3-[2-pyridyldithio]-propionate. Ca2+-saturated platelet-derived polyphosphate was a kind gift from Prof T. Renné (Karolinska Institute). Phospholipids (microparticle reagent, ∼20% phosphatidylserine and 80% phosphatidylcholine) were from Thrombinoscope (Maastricht, The Netherlands). Phosphatase was purchased from Sigma-Aldrich (St. Louis, MO) according to instructions from Prof Renné. All other chemicals were from Sigma-Aldrich.
Blood sampling and plasma preparation
After informed consent and permission by the local ethical committee, blood was drawn from healthy volunteers and from donors with a deficiency of FXII (undetectable FXII levels) and collected in 7.5-mL S-Monovette tubes (Sarstedt, Nümbrecht, Germany) with 1/10 (vol/vol) citrate (130 mM). Platelet-rich plasma was obtained by centrifugation at 180g for 20 minutes and platelet-free plasma by centrifugation at 2500g for 20 minutes followed by filtration through a 0.20-μm Minisart filter (Sartorius, Surrey, United Kingdom). Mouse blood was drawn from anesthetized mice by heart puncture into syringes with 1/10 (vol/vol) citrate (130 mM). All procedures in mice were conducted in accordance with the National Committee for Animal Research in Sweden and Principles of Laboratory Animal Care (National Institutes of Health Publication 86-23, revised 1985). The study was approved by the Local Ethics Committee for Animal Care and Use at Linköping University.
Whole blood and plasma clotting assays
Clotting times of recalcified whole blood and plasma were measured with a ReoRox4 rheometer (Medirox, Nyköping, Sweden),18 which detects the viscoelastic changes in clotting blood/plasma by measuring frequency and damping of the freely oscillating cup that contains the measured blood/plasma, or the coagulation instrument Amelung KC4 (Sigma-Aldrich) that uses a moving metal ball to detect fibrin formation in clotting blood/plasma. Citrated platelet-rich plasma was treated with platelet agonists or inhibitors. After a 5-minute incubation, platelet-poor plasma was obtained from these samples by centrifugation at 2500g for 10 minutes, and the clotting time of the plasma supernatant was measured after recalcification in a ReoRox4.
Citrated whole blood samples from normal and FXII-deficient individuals were treated with the well-known FXII activator celite (5 mg/mL) and the synthetic platelet activator A23187 (5 μM). The inhibitors annexin V (1 μg/mL) and CTI (100 μg/mL) were used with A23187 and celite activated samples. Extrinsic pathway coagulation was activated slightly with 0.6 pM TF (Innovin) prior to recalcification in some experiments.
FXII-dependent coagulation in the Amelung instrument was evaluated by measuring clotting times for human whole blood with resting or activated platelets (Trap6, 30 µM) and platelet-free plasma with added phospholipids (2.5 µM) (clotting was not detected for 2 donors in platelet-free plasma and were therefore excluded), with or without FXIIa inhibitor (CTI, 50 µg/mL) or phosphatase (10 U/mL, only for Trap6-activated samples).
FXIIa generation in platelet-rich and platelet-free plasma
Fluorogenic FXIIa substrate Boc-Gln-Gly-Arg-AMC was used to measure FXIIa generation in citrated human platelet-rich plasma. Platelets were activated using 30 μM Trap6, 300 μM PAR4-AP, or 2.5 μg/mL CRP. The positive control for FXII activation consisted of 3 glass beads (diameter = 1.1 mm). FXIIa was inhibited with 100 μg/mL CTI. Agonists, CTI, and beads were added to a 96-well plate, and the experiment was started with distribution of 100-µL aliquots of platelet-rich plasma with 80 µM FXIIa substrate to the plate. Evaluation of FXII activation by polyP was performed in the FXIIa generation assay by measuring fluorescence after cleavage of the FXIIa substrate Boc-Gln-Gly-Arg-AMC (80 µM) in the presence of 0, 10, 100, and 500 μg/mL of platelet polyP or kaolin in physiological saline. Fluorescence was measured in a Fluoroskan Ascent plate reader (Thermo Electron, Waltham, MA) at λex: 360 nm and λem: 460 nm for 30 minutes.
Evaluation of mouse blood in vitro
Mouse platelet response to thrombin receptor activating peptides Trap6 (PAR1 receptor) and PAR4-AP (PAR4 receptor) was evaluated in an Amelung KC4 instrument after addition of Trap6 (30 μM), PAR4-AP (300 μM), or without additives. Flow cytometric detection of bound fibrinogen was performed using an antifibrinogen chicken antibody and additions of Trap6 (30 μM), PAR4-AP (30 μM), or PAR4-AP (300 μM) using a Gallios flow cytometer (Beckman-Coulter, Miami, FL). Whole blood platelet aggregation was performed in a Multiplate instrument (Verum Diagnostica, Munich, Germany) with addition of Trap6 (30 μM) or PAR4-AP (170 μM).
Mouse model
Under isoflurane anesthesia, the jugular vein of male C57/BL-6 mice (n = 15 per group) was cannulated, and Trap6 (0.7, 2.8, and 11.2 µg/g body weight in the low, medium, and high dose groups, respectively), PAR4-AP (44.8 µg/g body weight), or saline was slowly infused. The allocation of mice to treatment group was random, coded, and blinded. Heart rate, breath rate, and oxygen saturation were monitored by pulse oximetry, and blood pressure was measured by tail-cuff volume pressure recording. After 40 minutes, spontaneously breathing animals were considered survivors and were euthanized by heart puncture. A more detailed account of the animal procedures is found in the supplemental Materials on the Blood website.
Statistical methods
Power calculations based on survival times were performed. With an α of 0.05, a least difference of interest of 15 minutes, an expected standard deviation of 10 minutes, and a power of ≥0.9, each group would have needed to include 9 animals, whereas our groups consisted of 15 animals. Survival time differences between treatment groups were analyzed by log-rank analysis, both in a total model and for combinations of the different groups. All other statistical testing was performed with a paired-samples t test, analysis of variance (ANOVA), or, where applicable, repeated-measures ANOVA with Tukey’s or Dunnett’s post hoc test. P < .05 was considered significant.
Results
Platelet polyP is a weak activator of coagulation FXII in vitro
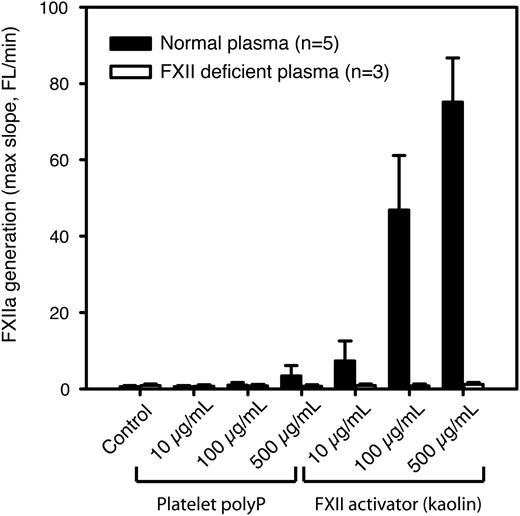
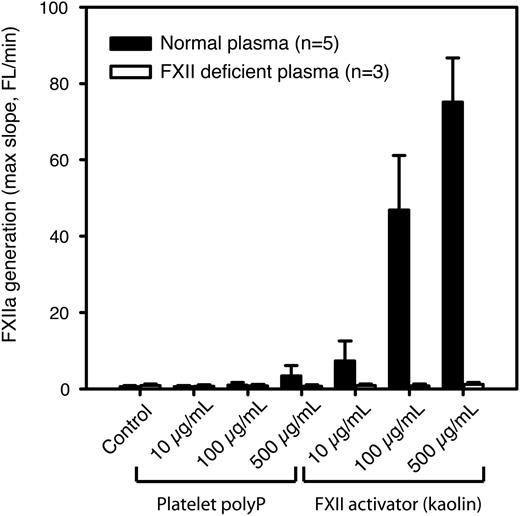
In paper A, it is stated that platelet-derived polyP is approximately equipotent to kaolin in supporting FXII activation. To verify this result, we used a fluorogenic substrate to detect activation of FXII by platelet-derived polyphosphates. Citrated platelet-free plasma was used to prevent nonspecific cleavage of the substrate by other coagulation factors downstream in the coagulation cascade, because the following steps in the cascade are all calcium dependent. In contrast to the results reported in paper A, our results show that the polyP preparation used in paper A exhibited an FXIIa-generating activity of <10% compared with equivalent concentrations of kaolin (Figure 1).
Platelet polyP is a much weaker activator of FXII than kaolin. FXIIa generation in citrated human platelet-free plasma was measured using an FXIIa-sensitive fluorogenic substrate in the presence of different concentrations of platelet polyP or kaolin (n = 5 normal and 3 FXII-deficient donors). Substrate fluorescence was measured for 30 minutes, and the maximum slope (from 5 neighboring data points) was calculated and is presented as mean ± standard deviation (SD).
Platelet polyP is a much weaker activator of FXII than kaolin. FXIIa generation in citrated human platelet-free plasma was measured using an FXIIa-sensitive fluorogenic substrate in the presence of different concentrations of platelet polyP or kaolin (n = 5 normal and 3 FXII-deficient donors). Substrate fluorescence was measured for 30 minutes, and the maximum slope (from 5 neighboring data points) was calculated and is presented as mean ± standard deviation (SD).
Platelets do not activate FXII
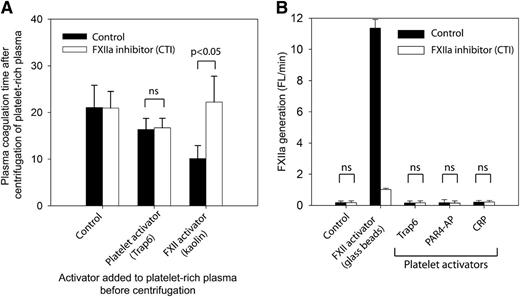
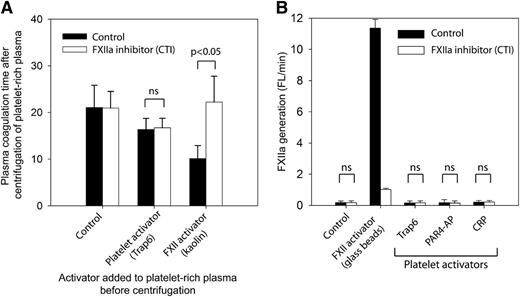
To validate the finding in paper A that activated platelets generate physiologically relevant amounts of FXIIa by the release of polyP, we measured the FXII dependency of clotting mechanisms in plasma after platelet activation and removal (Figure 2A). In brief, citrated platelet-rich plasma, with or without the FXII inhibitor CTI, was treated with (1) platelet inhibitors abciximab and prostaglandin I2, (2) platelet activator Trap6, or (3) a direct FXII activator (kaolin). Platelets were then removed by a centrifugation step. The coagulation times of the recalcified plasma supernatants were subsequently measured, and as expected, kaolin shortened the coagulation time significantly. The shortening was completely reversed on FXIIa inhibition by CTI (P < .05), clearly demonstrating an FXII-dependent mechanism. In contrast, activation by Trap6 in the absence of kaolin caused an FXII-independent shortening of coagulation time, because it was not reversed by CTI. These results show that platelets do not release detectable amounts of FXII-activating substances into citrated plasma.
Stimulated platelets do not release substances that activate FXII. (A) Citrated human platelet-rich plasma was either activated with platelet activator Trap6 (30 μM) or by kaolin (5 μg/mL) or treated with platelet inhibitors: aspirin (100 μM), prostaglandin I2 (1 μM), and abciximab (10 μg/mL) (Control). Platelets were removed from platelet-rich plasma by centrifugation at 2500g for 10 minutes, and the spontaneous clotting time after recalcification was measured (n = 3). (B) FXIIa generation in activated human platelet-rich plasma was measured by cleavage of a fluorogenic FXIIa substrate (n = 4). The platelets were either resting (control) or activated with Trap6 (30 µM), PAR4-AP (300 µM), or CRP (2.5 μg/mL). Glass beads in platelet-rich plasma were used as positive control. FXIIa activity was inhibited by addition of CTI (100 µg/mL). Measurements represent the average slope (fluorescence, arbitrary units/min) during 30 minutes and are presented as mean ± SD. Statistical testing was performed with ANOVA and Tukey’s post hoc test.
Stimulated platelets do not release substances that activate FXII. (A) Citrated human platelet-rich plasma was either activated with platelet activator Trap6 (30 μM) or by kaolin (5 μg/mL) or treated with platelet inhibitors: aspirin (100 μM), prostaglandin I2 (1 μM), and abciximab (10 μg/mL) (Control). Platelets were removed from platelet-rich plasma by centrifugation at 2500g for 10 minutes, and the spontaneous clotting time after recalcification was measured (n = 3). (B) FXIIa generation in activated human platelet-rich plasma was measured by cleavage of a fluorogenic FXIIa substrate (n = 4). The platelets were either resting (control) or activated with Trap6 (30 µM), PAR4-AP (300 µM), or CRP (2.5 μg/mL). Glass beads in platelet-rich plasma were used as positive control. FXIIa activity was inhibited by addition of CTI (100 µg/mL). Measurements represent the average slope (fluorescence, arbitrary units/min) during 30 minutes and are presented as mean ± SD. Statistical testing was performed with ANOVA and Tukey’s post hoc test.
It is, however, theoretically possible that released substances were retained on platelet membranes, although no such mechanism has been described. Although this concept would be largely in conflict with the actual preparation procedure of platelet polyP as described in paper A, where platelets were activated and then removed, similar to the experiment described above, we performed the following experiments to exclude this potential confounder. FXIIa generation was investigated in the presence of resting or activated platelets by measuring the cleavage of a fluorogenic FXIIa substrate in citrated platelet-rich plasma (Figure 2B). Platelet activators in this experiment were Trap6, PAR4-AP, or CRP. Glass beads were used as a positive control of FXIIa generation. The results show that FXIIa generation, by resting or activated platelets, was absent or extremely low, and no significant differences between samples with or without FXIIa inhibitor (CTI) were found. However, the glass that served as a positive control of contact activation clearly generated large quantities of FXIIa, a process that was inhibited by the addition of CTI. With this in mind, the platelet surface cannot be regarded as an efficient FXII activator in citrated plasma, especially if we consider that the approximated surface area of the platelets in this experiment is ∼75 times larger than that of the glass beads. The surface of platelet-rich clots as a possible locale of FXII activation was further dismissed by showing that FXII-dependent mechanisms have a marginal and platelet-independent effect on thrombin generation on preformed clots of different compositions (supplemental Figure 1). These results exclude a role for platelet-derived polyP as an initiator of FXII-dependent coagulation in citrated platelet-rich plasma.
Platelet contribution to whole blood coagulation is not FXII dependent
In paper A, the contribution of platelet-derived polyP to the initiation phase of FXII-dependent coagulation was investigated using the Amelung instrument, in which a moving metal ball is used for mechanical clot detection. It was reported that prestimulation of human platelets with the agonists A23187 (a calcium ionophore) or Trap6 both resulted in a 3.2-fold reduction of clotting times on recalcification, whereas incubation with phosphatase abrogated this effect. Clotting times of FXII-deficient samples were not reduced on preincubation with platelet agonists.12
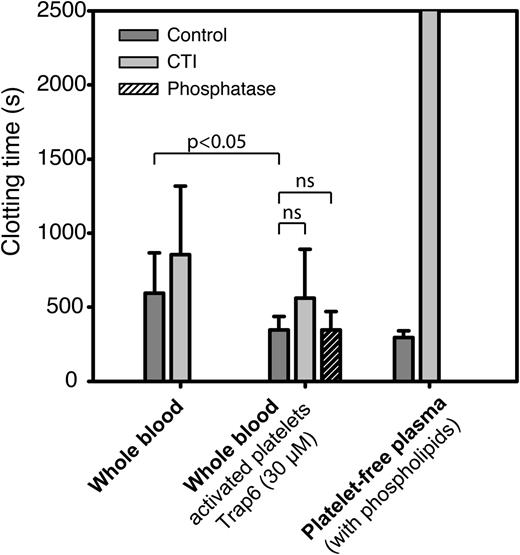
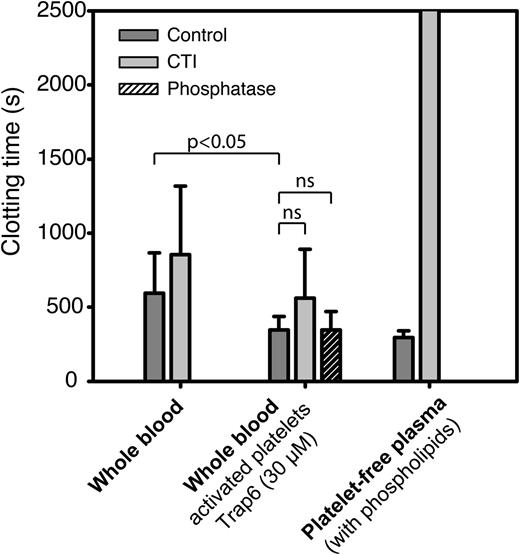
Using the same experimental conditions as in paper A, we compared recalcification times of whole blood, untreated or preactivated with the PAR1 agonist Trap6, to that of platelet-free plasma with added phospholipids. To assess the impact of polyP and FXIIa on clotting times, we also performed these experiments in the presence of phosphatase and CTI. Stimulation with Trap6 produced a significant (36%) shortening of clotting times, which was not reversed on preincubation with phosphatase (Figure 3). Inhibition of FXII resulted in a nonsignificant (32%) prolongation of clotting times in human whole blood pretreated with Trap6, but clotting times were equally prolonged (34%) on FXII inhibition of whole blood without preactivation of platelets (nonsignificant difference determined with paired-samples t test). Surprisingly, the clotting times recorded for platelet-free plasma with added phospholipids displayed a much more profound FXII dependence. These results indicate that the shortening of clotting times observed on preactivation of platelets with Trap6 in this assay is not dependent on polyP and that the sources of FXIIa that initiate FXII-dependent clotting in the Amelung instrument are not platelet derived.
In vitro clotting times in the Amelung instrument are more FXIIa dependent in platelet-free plasma containing phospholipids than in whole blood. Clotting times for human whole blood with resting or activated platelets (Trap6, 30 µM) and platelet-free plasma with added phospholipids (2.5 µM) to substitute for negatively charged platelet membranes were measured with or without FXIIa inhibitor (CTI, 50 µg/mL) or phosphatase (10 U/mL, purchased in accordance with instructions from Prof Renné). Data are presented as mean ± SD (whole blood: n = 10, platelet-free plasma: n = 3), and statistical testing was performed with repeated-measures ANOVA and Dunnett’s post hoc test for whole blood experiments.
In vitro clotting times in the Amelung instrument are more FXIIa dependent in platelet-free plasma containing phospholipids than in whole blood. Clotting times for human whole blood with resting or activated platelets (Trap6, 30 µM) and platelet-free plasma with added phospholipids (2.5 µM) to substitute for negatively charged platelet membranes were measured with or without FXIIa inhibitor (CTI, 50 µg/mL) or phosphatase (10 U/mL, purchased in accordance with instructions from Prof Renné). Data are presented as mean ± SD (whole blood: n = 10, platelet-free plasma: n = 3), and statistical testing was performed with repeated-measures ANOVA and Dunnett’s post hoc test for whole blood experiments.
Because these results demonstrate that the Amelung instrument did not detect any platelet-derived FXII activation and demonstrated artifactual FXII-dependent clotting in the absence of platelets, we considered the method not well suited for the task. For the assessment of the hypothetical contribution of activated platelets to the initiation phase of coagulation via activation of FXII, an alternative method, better fitted to adequately test this hypothesis, was sought. Such a method would ideally be unaffected by contact activation in the absence of activated platelets while still being highly sensitive to nonartifactual FXIIa generation. We hypothesized that adding a small amount of TF (0.6 pM) to a sensitive coagulation assay with minimal spontaneous generation of FXIIa could serve the double purpose of masking artifactual FXII activation while allowing for sensitive detection of FXII-dependent clotting caused by potential nonartifactual sources such as platelets. As evidenced by supplemental Figure 2, we found that the ReoRox4 instrument fulfilled these criteria, as it, even in the presence of TF, detected a significant difference (P = .011, n = 7) in clotting times after addition of the FXII activator SiO2 in a 10 times lower concentration range than the Amelung instrument used in paper A and displayed an unchanged clotting time on addition of the FXIIa inhibitor CTI to nonactivated blood samples (Figure 4). As expected, preincubation with A23187 (the platelet agonist used in corresponding experiments in paper A) resulted in a shortened clotting time, illustrating the well-known procoagulant activity of activated platelets. Addition of a small amount of TF also shortened the spontaneous clotting time, which could, in nonactivated samples, be very long in the ReoRox4 and thereby generate large standard deviations and in some case even no detectable clotting, making comparisons difficult. Other coagulation instruments with viscoelastic detection, eg, thromboelastography and rotational thromboelastometry, may also have been appropriate for the measurements, if proven sufficiently sensitive to FXII activation, but at the time of the study, these instruments were not available in the laboratory.
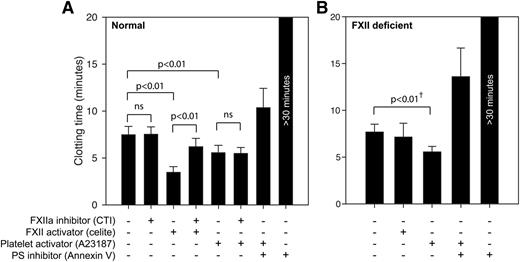
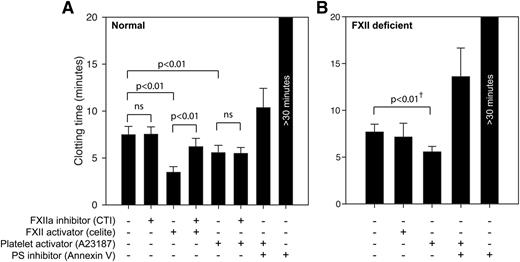
Human platelets do not shorten clotting times by FXII-activating mechanisms. Whole blood coagulation times were measured with the ReoRox instrument, using (A) normal whole blood and (B) FXII-deficient whole blood. A low concentration of TF was added to all samples to minimize the influence of material-induced FXII activation. Samples were either native or activated with the FXII activator celite (5 mg/mL) or platelet activator A23187 (5 μM). CTI (100 μg/mL) or annexin V (1 μg/mL) was used to inhibit the procoagulant activity of FXIIa and negatively charged phospholipids, respectively. Whole blood experiments in normal blood (n = 6-9) and from 2 FXII-deficient individuals are presented as mean ± SD. Statistical testing was performed with ANOVA and Tukey’s post hoc test for normal donors. †Statistical testing of FXII-deficient donors were performed with a paired-samples t test on each donor individually, using 8 repeated measurements per donor.
Human platelets do not shorten clotting times by FXII-activating mechanisms. Whole blood coagulation times were measured with the ReoRox instrument, using (A) normal whole blood and (B) FXII-deficient whole blood. A low concentration of TF was added to all samples to minimize the influence of material-induced FXII activation. Samples were either native or activated with the FXII activator celite (5 mg/mL) or platelet activator A23187 (5 μM). CTI (100 μg/mL) or annexin V (1 μg/mL) was used to inhibit the procoagulant activity of FXIIa and negatively charged phospholipids, respectively. Whole blood experiments in normal blood (n = 6-9) and from 2 FXII-deficient individuals are presented as mean ± SD. Statistical testing was performed with ANOVA and Tukey’s post hoc test for normal donors. †Statistical testing of FXII-deficient donors were performed with a paired-samples t test on each donor individually, using 8 repeated measurements per donor.
Under these conditions, the clotting time induced by the direct FXII activator celite was significantly prolonged (P < .001) on addition of the FXII inhibitor (CTI). However, platelet procoagulant activity induced by the calcium ionophore A23187 was unaffected by FXII inhibition while being strongly suppressed when membrane phosphatidylserine was blocked by annexin V. Corresponding coagulation experiments with samples from 2 FXII-deficient donors were conducted to verify these FXII-independent effects (Figure 4B). In FXII-deficient samples, activation with a direct FXII activator (celite) produced no shortening of clotting time, whereas platelet activation by a calcium ionophore (A23187) induced a shortening of the clotting time in an extent similar to that in normal donors. Statistical testing (paired-samples t test) for each of the FXII-deficient donors comparing control and A23187-treated samples (using 3 measurements for each treatment and donor) demonstrated a significant shortening (P < .05) of coagulation times by an average of 28% due to calcium ionophore treatment, whereas celite had no significant effect.
Key experimental findings in paper A supporting a role for platelet-derived polyphosphates in thrombogenesis in vivo are irreproducible
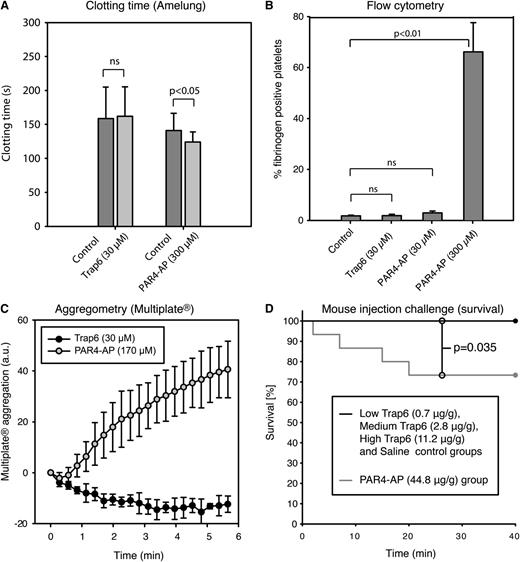
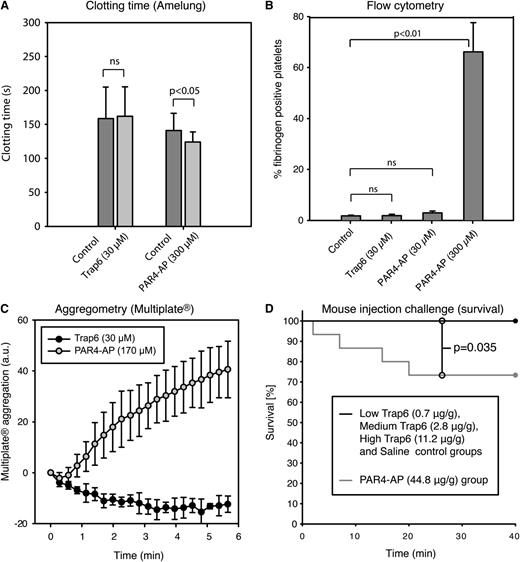
In support for a role of platelet-derived polyphosphates in coagulation, Müller et al12 reported that infusion of Trap6, 0.7 µg/g body weight, in wild-type mice induced lethal pulmonary embolism, but FXII−/− mice were protected. Pretreatment with platelet inhibitors or phosphatase also rescued wild-type mice. Correspondingly, pretreatment of mouse platelets with Trap6 was stated to produce a 2.6-fold reduction of clotting times. To verify these findings, we performed clotting tests, analyzed platelet activation markers by flow cytometry, and studied whole blood aggregation in blood collected from mice of the same strain as was used in paper A. Flow cytometry revealed no platelet activation measured as fibrinogen binding (Figure 5B). Further, Trap6 failed to induce aggregation or reduce clotting times in mouse blood (Figure 5A,C). In all mouse experiments, PAR4-AP was used as a positive control, because PAR4 is the receptor principally responsible for thrombin-induced platelet activation in mice.15,16 In an attempt to understand how and why the mice in the experiments presented in paper A died within 30 minutes of injection of 0.7 µg Trap6/g body weight, we repeated these experiments. No animals died in our low (0.7 µg Trap6/g body weight), medium (2.8 µg Trap6/g body weight), or high (11.2 µg Trap6/g body weight) Trap6 groups, nor in the saline control group within the 40-minute duration of the experiment (Figure 5D); moreover, the mice displayed no differences in oxygen saturation, heart rate, or mean arterial blood pressure (supplemental Figure 3). In the PAR4-AP group, which was used as the positive control, 4 of 15 animals died, significantly more than in the other groups (P = .035; Figure 5D). The PAR4-AP group also showed significantly lower average oxygen saturation than the other groups (P < .05) (supplemental Figure 3). The person performing and evaluating the animal experiments in our study was blinded to the substance injected.
Mouse platelets do not respond to Trap6 (PAR1-activating peptide), and vascular injection of Trap6 is not lethal to mice. The mouse platelet response to Trap6 was evaluated using (A) whole blood clotting time (n = 5, individual controls were used for the 2 peptides because of limited blood volume), (B) platelet fibrinogen binding detected by flow cytometry in citrated blood (n = 3), and (C) citrated whole blood platelet aggregation (using Multiplate; n = 4). No platelet activation was detected on addition of 30 µM Trap6 peptide. PAR4-AP was used as a positive control at 300/170 µM and led to considerable platelet activation in all experiments. It is well known that PAR4-AP is required at higher concentrations to achieve full activation. (D) Survival of mice that were challenged by injections with Trap6 or PAR4-AP into the jugular vein. In the group receiving 44.8 µg PAR4-AP/g body weight, 4 of 15 animals died, whereas none died in the groups receiving Trap6 (low: 0.7 µg/g body weight; medium: 2.8 µg/g body weight; high: 11.2 µg/g body weight) or saline (n = 15 per group; P = .035 vs PAR4-AP). The x-axis represents time in minutes after administration of substance. All measurements (except in D) are presented as mean ± SD. Statistical testing was performed with (A-B) ANOVA and Tukey’s post hoc test, and (D) survival time differences between treatment groups were analyzed by log-rank analysis, both in a total model and for combinations of the different groups.
Mouse platelets do not respond to Trap6 (PAR1-activating peptide), and vascular injection of Trap6 is not lethal to mice. The mouse platelet response to Trap6 was evaluated using (A) whole blood clotting time (n = 5, individual controls were used for the 2 peptides because of limited blood volume), (B) platelet fibrinogen binding detected by flow cytometry in citrated blood (n = 3), and (C) citrated whole blood platelet aggregation (using Multiplate; n = 4). No platelet activation was detected on addition of 30 µM Trap6 peptide. PAR4-AP was used as a positive control at 300/170 µM and led to considerable platelet activation in all experiments. It is well known that PAR4-AP is required at higher concentrations to achieve full activation. (D) Survival of mice that were challenged by injections with Trap6 or PAR4-AP into the jugular vein. In the group receiving 44.8 µg PAR4-AP/g body weight, 4 of 15 animals died, whereas none died in the groups receiving Trap6 (low: 0.7 µg/g body weight; medium: 2.8 µg/g body weight; high: 11.2 µg/g body weight) or saline (n = 15 per group; P = .035 vs PAR4-AP). The x-axis represents time in minutes after administration of substance. All measurements (except in D) are presented as mean ± SD. Statistical testing was performed with (A-B) ANOVA and Tukey’s post hoc test, and (D) survival time differences between treatment groups were analyzed by log-rank analysis, both in a total model and for combinations of the different groups.
In paper A, it was also reported that infusion of 300 µg/g body weight polyphosphate in wild-type mice induced lethal pulmonary embolism. Assuming an average mouse weight of 25 g and a blood volume of 1.5 mL, this is equivalent to a concentration of ∼5000 µg/mL in mouse blood. However, we found that the platelet-derived polyphosphate preparation kindly supplied by the authors of paper A was not possible to dissolve at concentrations as low as 780 µg/mL in physiological saline (the solvent used in paper A), as shown by the appearance of polyphosphate-containing particles with a size equal to or bigger than platelets on micrographs of suspensions containing polyphosphates at different concentrations (supplemental Figure 4). Staining with 4′,6-diamidino-2-phenylindole was used to verify polyP content, because this dye was used in paper A and elsewhere12 for detection of polyP.
Discussion
In paper A, it is claimed that “PolyP represents the long sought ‘foreign’ surface that triggers fibrin formation by activated platelets linking primary to secondary hemostasis and critically contributing to ‘procoagulant’ platelet activity.”12 If correct, the assertion that platelets induce FXII-driven coagulation by the release of polyphosphates would indeed represent a paradigmatic shift in our conceptual understanding of thrombogenesis and provide an eloquent synthesis of 2 emerging areas in hemostasis research. However, the theoretical appeal of a certain hypothesis cannot serve as a surrogate for the demand of reproducibility. Our findings do not support the notion that platelet-derived polyphosphates are physiologically relevant activators of FXII because (1) platelets do not release physiologically relevant amounts of FXII-activating substances on activation, nor do they express such substances on the platelet membrane; (2) platelet-derived polyphosphates are weak activators of FXII, with a FXIIa-generating activity of <10% compared with equivalent concentrations of kaolin; and (3) platelet contribution to coagulation is not mediated by FXIIa generation. We also show that key results from in vivo experiments reported by the proponents of this theory are irreproducible because (4) infusion of Trap6 does not cause activation of murine platelets; and (5) the polyphosphates used in the original experiments are not soluble at concentrations that would allow for infusion of the stated amounts into mice in a nonparticulate state. Interestingly, one of the coauthors of paper A has recently provided support for our observation regarding the FXIIa-generating capacity of platelet-derived polyphosphates, reporting that platelet polyP chain length is too short to efficiently activate FXII.19
In contrast to Müller et al,12 we found that preincubation with phosphatase did not reverse Trap6-dependent reduction of clotting times, suggesting that this effect is not caused by polyphosphates. As phosphatases are known to have other nonspecific activities, eg, inhibition of platelet activation by degradation of ADP,20 we verified these findings using the well-known FXII inhibitor CTI. We found that FXII contributed to clotting to an equal extent in samples with or without preactivation of platelets, and much more so when platelets were substituted by phospholipids in the assay used in paper A. This finding suggests that FXIIa generation occurred independently of platelets in the Amelung instrument, whereas FXIIa-dependent clotting was amplified in the presence of phospholipids, of which the latter observation is supported by previous findings.21 Although the exact mechanisms of FXIIa generation in the Amelung assay were not identified, it is, in this context, worth reiterating the well-known fact that in every conventional in vitro assay used to monitor coagulation, small, but not negligible, amounts of FXII will eventually be generated by plastic or metal surfaces in contact with plasma.22-25 In the absence of strong activators of coagulation, such artifactual sources of FXIIa will become the predominant procoagulant mechanism. Thus, the identity of the “foreign surface” triggering generation of FXIIa, as referred to in the above quote, is far more likely to have been the moving metal ball used to monitor coagulation in the Amelung instrument than platelet-derived polyphosphates.
Observations supporting a role for platelets in contact activation and the intrinsic pathway of coagulation have been reported for >4 decades. In one of the most notable contributions on this subject, Walsh and Griffin26 reported that platelets appear to bind FXII and thereby facilitate cleavage of FXII by kallikrein in the presence of high-molecular-weight kininogen. However, activation of platelets with ADP or collagen in the absence of kallikrein was not reported to generate detectable amounts of FXIIa in a sensitive radiometric assay, supporting the findings in this study. Curiously, the well-described FXII-independent procoagulant activity of phosphatidylserine-exposing platelets,27-29 previously referred to as platelet factor 3, was not detected in the assay used in paper A, because platelet activation was reported to have no impact on clotting times on recalcification in FXII-deficient samples. Using the Reorox instrument, which in control experiments proved to be more sensitive than the Amelung instrument in detecting FXII-dependent coagulation (supplemental Figure 2), we found that the shortening of clotting times observed on preactivation of platelets was independent of FXII, but highly dependent on the well-known procoagulant activity of stimulated platelets exposing negatively charged phospholipids.
We would like to stress that we do not dispute that platelet-derived polyP could play an important role at other stages in the coagulation cascade, for example, in accelerating the activation of factor V or factor XI by thrombin.30,31 Likewise, the findings reported in paper A regarding the proinflammatory effects of polyP have not been investigated in this study and are therefore not disputed herein. The putative role of FXII in thrombosis remains a promising new avenue for thrombosis research and bears the promise of new therapeutic approaches to thromboembolic disease. Alternative loci for activation of FXII in vivo have been proposed, such as the injured vessel wall or neutrophil extracellular traps.1,9-11,32 Hopefully, the present data will provide guidance in the ongoing effort to unveil the role of FXII in thrombosis.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was funded by Swedish Research Council project 521-2012-2729, Swedish Heart and Lung Foundation project 20100219, and Biomaterials and Cell Therapy center of excellence at University of Gothenburg funded by Swedish Governmental Agency for Innovation Systems.
Authorship
Contribution: L.F. performed research, contributed to the design of the study, carried out interpretation of data, and wrote the paper; N.B. performed research, carried out interpretation of data, and wrote the paper; J.O.S. participated in the design and performance of the animal experiments; P.T. contributed with specific areas of research and revision of the manuscript; E.T. contributed to the design and interpretation of animal experiments and revision of the manuscript; S.R. contributed to data interpretation and manuscript revision; and T.L.L. participated in the design of the study, carried out interpretation of data, and wrote the paper.
Conflict of interest disclosure: T.L.L. and E.T. are members of the board and minor shareholders of Medirox (E.T. only since April 25, 2013). The other authors declare no competing financial interests.
Correspondence: Tomas Lindahl, Department of Clinical and Experimental Medicine, Linköping University, SE-58185 Linköping, Sweden; e-mail: tomas.lindahl@liu.se.